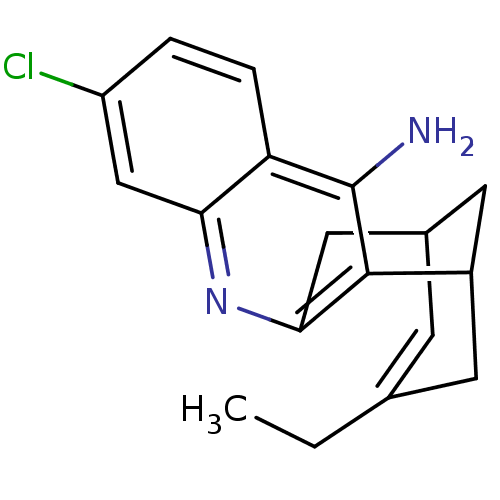
BDBM10597 (1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{2,11}.0^{4,9}]heptadeca-2(11),3,5,7,9,14-hexaen-3-amine::7-chloro-15-ethyl-(1R,13S)-10-azoniatetracyclo[11.3.1.02,11.04,9]heptadeca-2,4,6,8,10,14-hexaen-3-amine::CHEMBL143812::Huprine X::rac-huprine H7
SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1N
InChI Key InChIKey=QTPHSDHUHXUYFE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 10597
Found 6 hits for monomerid = 10597
Affinity DataKi: 0.0260nM ΔG°: -14.4kcal/molepH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c...More data for this Ligand-Target Pair
Affinity DataKi: 0.0260nM ΔG°: -14.4kcal/molepH: 7.0 T: 2°CAssay Description:Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we...More data for this Ligand-Target Pair
Affinity DataKi: 120nM ΔG°: -9.43kcal/molepH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c...More data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMAssay Description:Inhibitory activity against human erythrocyte acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.77nMAssay Description:Inhibition of bovine erythrocyte AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:In vitro inhibitory activity against bovine acetylcholinesteraseMore data for this Ligand-Target Pair