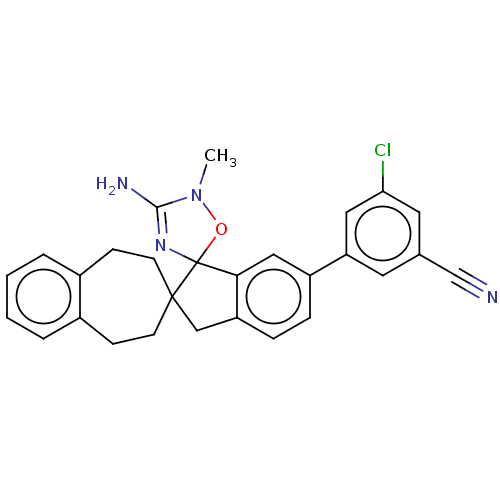
BDBM113232 US10336717, Compound 10::US8633212, 10::US9212153, 10
SMILES CN1OC2(N=C1N)c1cc(ccc1CC21CCc2ccccc2CC1)-c1cc(Cl)cc(c1)C#N
InChI Key InChIKey=BSLOJIAKFHBYMO-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 113232
Found 3 hits for monomerid = 113232
TargetIsoform C of Beta-secretase 1 (BACE-I-457)(Homo sapiens (Human))
Vitae Pharmaceuticals
US Patent
Vitae Pharmaceuticals
US Patent
Affinity DataIC50: 7.40nMpH: 4.5 T: 2°CAssay Description:Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity. The assay was performed at room temperature in 96-well...More data for this Ligand-Target Pair
Affinity DataIC50: 7.40nMAssay Description:For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fuse...More data for this Ligand-Target Pair
Affinity DataIC50: 7.40nMpH: 4.5 T: 2°CAssay Description:Inhibitory activity of compounds was assessed by a fluorescence quench assay of BACE activity using commercially available substrate HiLyte Fluor 488...More data for this Ligand-Target Pair