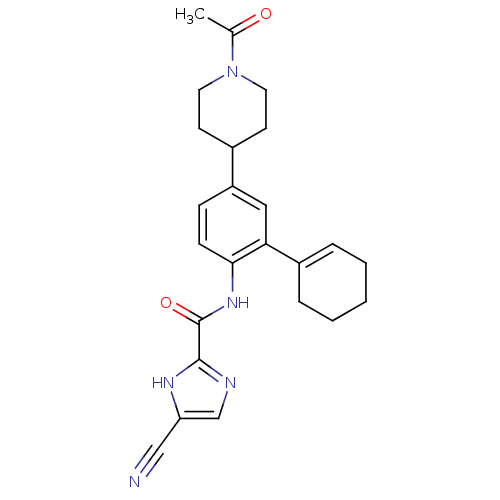
BDBM120076 CHEMBL256712::US8697716, 16
SMILES CC(=O)N1CCC(CC1)c1ccc(NC(=O)c2ncc([nH]2)C#N)c(c1)C1=CCCCC1
InChI Key InChIKey=UKGCAEYEQULSSN-UHFFFAOYSA-N
Data 9 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 120076
Found 9 hits for monomerid = 120076
TargetMacrophage colony-stimulating factor 1 receptor(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of FMS mediated phosphorylation using SYEGNSYTFIDPTQ as substrate after 80 mins by fluorescence polarizationMore data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Mouse)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 5.60nMAssay Description:Inhibition of FMS-mediated proliferation in CSF1-stimulated bone marrow-derived mouse macrophages assessed as inhibition of incorporation of bromodeo...More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetCytochrome P450 3A4(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: >3.20E+3nMAssay Description:Displacement of [3H]astemizole from human ERG potassium channelMore data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:The compounds of the present invention are also specific inhibitors of c-Kit. Selection of preferred compounds of Formula I for use as c-Kit inhibito...More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 9.20E+3nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair