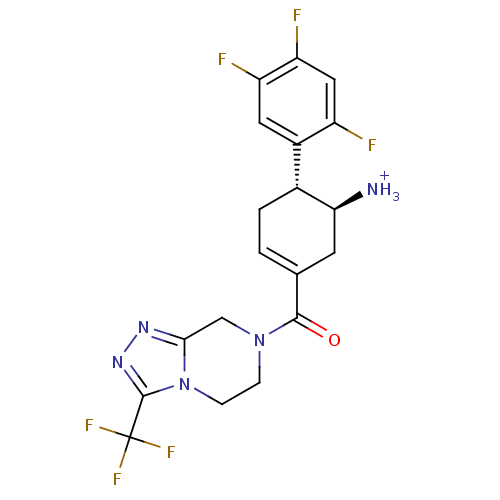
BDBM12656 ((4R,5S)-5-Amino-4-(2,4,5-trifluorophenyl)cyclohex-1-enyl)-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone::(1S,6R)-3-{[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]carbonyl}-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium trifluoroacetate::(1S,6R)-3-{[3-(trifluoromethyl)-5H,6H,7H,8H-[1,2,4]triazolo[3,4-a]pyrazin-7-yl]carbonyl}-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate::ABT-341
SMILES [NH3+][C@H]1CC(=CC[C@@H]1c1cc(F)c(F)cc1F)C(=O)N1CCn2c(C1)nnc2C(F)(F)F
InChI Key InChIKey=NVVSPGQEXMJZIR-BMIGLBTASA-O
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 12656
Found 3 hits for monomerid = 12656
Affinity DataKi: 1.30nM ΔG°: -12.0kcal/molepH: 7.5 T: 2°CAssay Description:The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas...More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nM ΔG°: -7.28kcal/molepH: 7.5 T: 2°CAssay Description:The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas...More data for this Ligand-Target Pair
Affinity DataKi: >3.00E+4nM ΔG°: >-6.10kcal/molepH: 7.5 T: 2°CAssay Description:The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas...More data for this Ligand-Target Pair