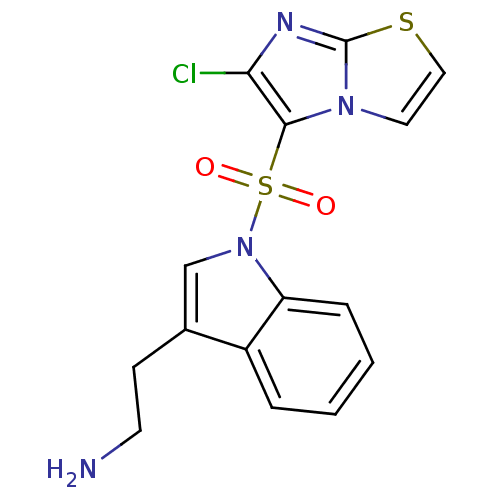
BDBM21358 2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulfonyl)-1H-indol-3-yl]ethan-1-amine::N1-(6-chloroimidazo[2,1-b][1,3]thiazole-5-sulfonyl)tryptamine::SAX-187::WAY-181187
SMILES NCCc1cn(c2ccccc12)S(=O)(=O)c1c(Cl)nc2sccn12
InChI Key InChIKey=RYBOXBBYCVOYNO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 21358
Found 16 hits for monomerid = 21358
Affinity DataKi: 2nM ΔG°: -11.7kcal/mole EC50: 6.5nMpH: 7.4 T: 2°CAssay Description:IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H]lysergic acid diethylamide from human 5-HT6 receptor expressed in HeLa cells after 120 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 2nM ΔG°: -12.3kcal/mole EC50: 6.5nMpH: 7.4 T: 2°CAssay Description:Radioligand binding assays were performed using membranes from HEK-293 transfected with human 5-HT6 receptor. In these membranes the receptor concent...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Binding affinity to 5-HT6R (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 124nM ΔG°: -9.32kcal/molepH: 7.4 T: 2°CAssay Description:IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti...More data for this Ligand-Target Pair
Affinity DataKi: 458nM ΔG°: -8.55kcal/molepH: 7.4 T: 2°CAssay Description:IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti...More data for this Ligand-Target Pair
Affinity DataKi: 679nM ΔG°: -8.32kcal/molepH: 7.4 T: 2°CAssay Description:IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.96E+5nMpH: 7.4 T: 2°CAssay Description:The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra...More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMpH: 7.4 T: 2°CAssay Description:The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.12E+5nMpH: 7.4 T: 2°CAssay Description:The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+4nMpH: 7.4 T: 2°CAssay Description:The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.49E+5nMpH: 7.4 T: 2°CAssay Description:The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra...More data for this Ligand-Target Pair
Affinity DataEC50: 6.5nMAssay Description:Agonist activity at human 5HT6 receptor expressed in HeLa cells assessed as induction of cAMP production after 10 mins by radioimmunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+5nMpH: 7.4 T: 2°CAssay Description:The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra...More data for this Ligand-Target Pair