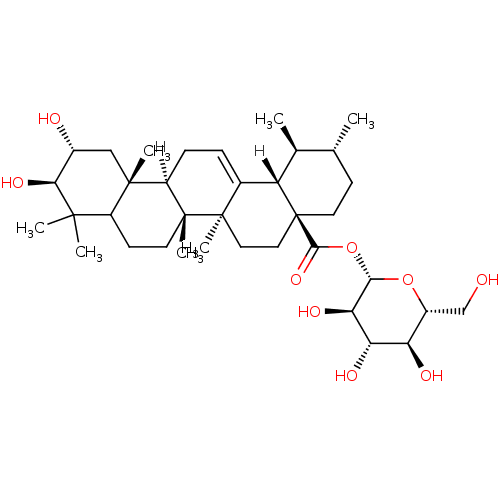
BDBM23206 (2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl (1S,2R,4aS,6aS,6bR,10R,11R,12aR,12bR,14bS)-10,11-dihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate::Corosolic acid beta-D-glucopyranosyl ester, 20
SMILES [H][C@]12CC=C3[C@]4([H])[C@@H](C)[C@H](C)CC[C@@]4(CC[C@@]3(C)[C@]1(C)CCC1C(C)(C)[C@@H](O)[C@H](O)C[C@]21C)C(=O)O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O
InChI Key InChIKey=HZKJZYRLLBKBHA-KSJNCMOLSA-N
Data 1 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 23206
Found 1 hit for monomerid = 23206
TargetGlycogen phosphorylase, muscle form(Oryctolagus cuniculus (rabbit))
China Pharmaceutical University
China Pharmaceutical University
Affinity DataIC50: 1.06E+5nMpH: 7.2 T: 2°CAssay Description:The activity of the compounds is determined by measuring the inhibitory effect of the compounds in the direction of glycogen synthesis, the conversio...More data for this Ligand-Target Pair