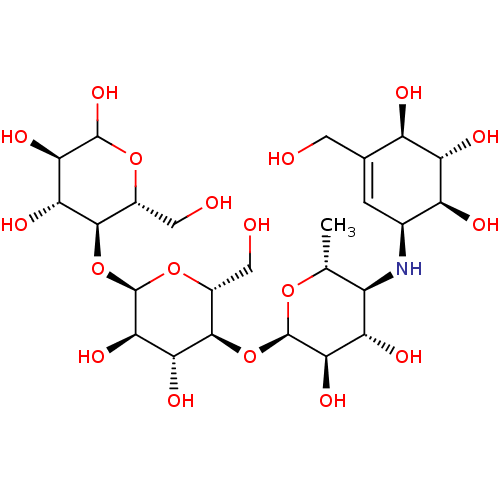
BDBM23406 (3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl-5-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl]amino}oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-6-(hydroxymethyl)oxane-2,3,4-triol::Acarbose::US11292789, Acarbose
SMILES C[C@H]1O[C@H](O[C@@H]2[C@@H](CO)O[C@H](O[C@@H]3[C@@H](CO)OC(O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C=C(CO)[C@@H](O)[C@H](O)[C@H]1O
InChI Key InChIKey=XUFXOAAUWZOOIT-UGEKTDRHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 77 hits for monomerid = 23406
Found 77 hits for monomerid = 23406
Affinity DataKi: 440nMAssay Description:Non-competitive inhibition of alpha glucosidase (unknown origin) assessed as inhibition constant using pNPG as substrate by by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 996nMpH: 6.0 T: 2°CAssay Description:The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b...More data for this Ligand-Target Pair
TargetOligo-1,6-glucosidase IMA1(Saccharomyces cerevisiae S288c (Baker's yeast))
University of The Punjab
University of The Punjab
Affinity DataIC50: 3.83E+4nMAssay Description:At 37 °C, after incubation of 10 min of a reaction mixture solution of 70 μL (50 mM) phosphate buffer of 6.8 pH, 10 μL (0.5 mM) of a test c...More data for this Ligand-Target Pair
TargetOligo-1,6-glucosidase IMA1(Saccharomyces cerevisiae S288c (Baker's yeast))
University of The Punjab
University of The Punjab
Affinity DataIC50: 3.83E+4nMpH: 6.8Assay Description:The α-glucosidase inhibition assay had been carried out using baker¿s yeast a-glucosidase (EC 3.2.1.20) and p-nitrophenyl-α-D-glucopyranosi...More data for this Ligand-Target Pair
TargetOligo-1,6-glucosidase IMA1(Saccharomyces cerevisiae S288c (Baker's yeast))
University of The Punjab
University of The Punjab
Affinity DataIC50: 7.75E+5nMpH: 6.8Assay Description:The enzyme inhibition was evaluated according to the method previously reported by Rahim et al. [25] with slight modification. Various concentration ...More data for this Ligand-Target Pair
TargetOligo-1,6-glucosidase IMA1(Saccharomyces cerevisiae S288c (Baker's yeast))
University of The Punjab
University of The Punjab
Affinity DataIC50: 9.37E+3nMAssay Description:α-Glucosidase inhibitory activity was evaluated at 37 °C, by using 0.1M phosphatebuffer (pH6.8) [28]. The enzyme(EC3.2.1.20, Saccharomyces cerev...More data for this Ligand-Target Pair
TargetOligo-1,6-glucosidase IMA1(Saccharomyces cerevisiae S288c (Baker's yeast))
University of The Punjab
University of The Punjab
Affinity DataIC50: 3.83E+4nMpH: 6.8Assay Description:The α-glucosidase inhibition activity was performed with slight modifications according to method. Total volume of 100 µL reaction mixture conta...More data for this Ligand-Target Pair
TargetOligo-1,6-glucosidase IMA1(Saccharomyces cerevisiae S288c (Baker's yeast))
University of The Punjab
University of The Punjab
Affinity DataIC50: 8.56E+5nMAssay Description:The 135 µL of 50 mM phosphate saline buffer pH (6.8), solution of enzyme (20 µL), and 20 µL of test sample with 70% DMSO was added to 96-well plate. ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.33E+4nMpH: 6.8Assay Description:The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed by u...More data for this Ligand-Target Pair
TargetMannosyl-oligosaccharide glucosidase(Saccharomyces cerevisiae (Yeast))
Recep Tayyip Erdogan University
Recep Tayyip Erdogan University
Affinity DataIC50: 1.33E+4nMpH: 6.8Assay Description:α-Glucosidase inhibition assay was performed spectrophotometrically. α-Glucosidase from Saccharomyces cerevisiae (Sigma-Aldrich) was dissol...More data for this Ligand-Target Pair
Affinity DataIC50: 8.17E+5nMpH: 6.8Assay Description:The test compounds were dissolved in DMSO to prepare the required distributing concentration. α-Glucosidase inhibitory activity was assayed usin...More data for this Ligand-Target Pair
Affinity DataIC50: 8.17E+5nMpH: 6.8 T: 2°CAssay Description:The α-glucosidase inhibition assay had been carried out using baker's yeast α-glucosidase (EC 3.2.1.20) and p-nitrophenyl α-d-gluco...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+3nMpH: 4.5Assay Description:α-Amylase activity was assayed with the chromogenic substrate RBB-starch. An enzyme aliquot was incubated (20 min, 26°C) with 0.3% RBB-starch in...More data for this Ligand-Target Pair
TargetOligo-1,6-glucosidase IMA1(Saccharomyces cerevisiae S288c (Baker's yeast))
University of The Punjab
University of The Punjab
Affinity DataIC50: 7.75E+5nMpH: 6.8 T: 2°CAssay Description:The enzyme inhibition was evaluated according to the method previously reported by Zawawi et al. [Zawawi et al., Bioorg. Chem., 23:3119] with slight ...More data for this Ligand-Target Pair
TargetOligo-1,6-glucosidase IMA1(Saccharomyces cerevisiae S288c (Baker's yeast))
University of The Punjab
University of The Punjab
Affinity DataIC50: 3.83E+4nMpH: 6.8 T: 2°CAssay Description:The enzyme assays were done in the initial linear parts of the assay curve with suitable positive and negative controls (data not shown). A total of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.31E+5nMpH: 6.8 T: 2°CAssay Description:Briefly, the sample solution (25 μL, 1.0 mM solution in DMSO) was mixed with enzyme solution (25 μL, 0.5 U/mL prepared in 0.1 M phosphate buffe...More data for this Ligand-Target Pair
Affinity DataIC50: 3.83E+4nMpH: 6.8 T: 2°CAssay Description:For α-glucosidase inhibition assay, α-glucosidase enzyme (Cat No. 5003-1KU Type I; isolated from Saccharomyces cereviciae) was used. Acarbo...More data for this Ligand-Target Pair
Affinity DataIC50: 9.08E+5nMpH: 7.0 T: 2°CAssay Description:α-glucosidase (25 μL, 0.2 U/mL), 25 μL of various concentrations of samples, and 175 μL of 50 mM sodium phosphate buffer (pH 7.0)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.75E+5nMpH: 7.0Assay Description:In this study, the inhibition assay of yeast enzyme was performed in 100 mM phosphate buffer pH 7.0 at 25°C with minor changes, according to the meth...More data for this Ligand-Target Pair
Affinity DataIC50: 1.36E+4nMpH: 7.0Assay Description:In this study, the inhibition assay of yeast enzyme was performed in 100 mM phosphate buffer pH 7.0 at 25°C with minor changes, according to the meth...More data for this Ligand-Target Pair
Affinity DataIC50: 2.63E+4nMAssay Description:Inhibition of hog pancreas alpha-amylase using starch as substrate preincubated for 10 mins followed by substrate addition measured after 10 mins by ...More data for this Ligand-Target Pair
TargetOligo-1,6-glucosidase IMA1(Saccharomyces cerevisiae S288c (Baker's yeast))
University of The Punjab
University of The Punjab
Affinity DataIC50: 8.17E+5nMAssay Description:Inhibition of bakers yeast alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 15 mins followed by substrate add...More data for this Ligand-Target Pair
TargetSucrase-isomaltase, intestinal(Rattus norvegicus (Rat))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.02E+4nMAssay Description:Inhibition of sucrase in rat small intestinal mucosa assessed as reduction in glucose production using sucrose as substrate measured after 40 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: 5.30E+3nMAssay Description:Inhibition of porcine pancreatic alpha-amylase using starch as substrate preincubated for 15 mins followed by substrate addition measured after 10 mi...More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+6nMAssay Description:Inhibition of recombinant Ruminococcus obeum ATCC 29174 alpha-glucosidase expressed in Escherichia coli BL21(DE3) using p-nitrophenyl-alpha-D-glucopy...More data for this Ligand-Target Pair
Affinity DataIC50: 8.17E+5nMAssay Description:Inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl-alpha-D-glucopyranoside as substrate preincubated for 15 mins followed by substr...More data for this Ligand-Target Pair
Affinity DataIC50: 8.93E+3nMAssay Description:Inhibition of porcine pancreatic alpha-amylase using starch as substrate after 30 mins by iodine reagent based assayMore data for this Ligand-Target Pair
TargetLysosomal alpha-glucosidase/Maltase-glucoamylase/Probable maltase-glucoamylase 2/Sucrase-isomaltase, intestinal(Homo sapiens (Human))
College Of Pharmacy/Guangdong Province Key Laboratory Of Pharmacodynamic Constituents Of Tcm And New Drugs Research
Curated by ChEMBL
College Of Pharmacy/Guangdong Province Key Laboratory Of Pharmacodynamic Constituents Of Tcm And New Drugs Research
Curated by ChEMBL
Affinity DataIC50: 4.42E+5nMAssay Description:Inhibition of alpha-glucosidase (unknown origin) using p-NPG as substrate pretreated for 10 mins followed by substrate addition measured after 5 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+5nMAssay Description:Inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl glycoside as substrate treated 20 mins post substrate addition measured for 1 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 1.35E+3nMAssay Description:Inhibition of porcine pancreatic alpha-amylase using starch as substrate preincubated for 10 mins followed by substrate addition measured after 10 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 7.08E+5nMAssay Description:Inhibition of human alpha-glucosidase expressed in Saccharomyces cerevisiae using p-nitrophenyl-alpha-D-glucopyranoside as substrate after 15 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+5nMAssay Description:Inhibition of alpha-glucosidase (unknown origin) using p-nitro-phenyl-alpha-D-glucopyranoside as substrate preincubated for 5 mins with substrate fol...More data for this Ligand-Target Pair
Affinity DataIC50: 9.42E+5nMAssay Description:Inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl-alpha-d-glucopyranoside as substrate preincubated for 15 mins followed by substr...More data for this Ligand-Target Pair
Affinity DataIC50: 8.17E+5nMAssay Description:Inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl-alpha-d-glucopyranoside as substrate preincubated for 15 mins followed by substr...More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+4nMAssay Description:Inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl alpha-D-glucoside as substrate preincubated for 0.5 hrs followed substrate addit...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of alpha-glucosidase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 8.17E+5nMAssay Description:Inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl alpha-d-glucopyranoside as substrate preincubated for 15 mins followed by substr...More data for this Ligand-Target Pair
Affinity DataIC50: 1.52E+4nMAssay Description:Inhibition of human intestinal maltase using maltose as substrate incubated for 30 mins and immediately heated for 2 mins by glucose oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 126nMAssay Description:Using the samples prepared according to Example 1 and Comparative Examples 2 and 3, inhibitory activity against α-glucosidase was evaluated to a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.66E+3nMAssay Description:Inhibition of porcine pancreatic alpha-amylase using 1% starch as substrate preincubated for 10 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 3.45E+4nMAssay Description:Inhibition of alpha-glucosidase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.44E+4nMAssay Description:Inhibition of alpha-glucosidase (unknown origin) using PNPG as substrate preincubated for 15 mins followed by substrate addition and measured after 1...More data for this Ligand-Target Pair
Affinity DataIC50: 5.84E+5nMAssay Description:Inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl-glycoside as substrate preincubated for 10 mins followed by substrate addition b...More data for this Ligand-Target Pair
Affinity DataIC50: 4.07E+4nMAssay Description:Inhibition of alpha-glucosidase (unknown origin) using pNPG as substrate preincubated with enzyme for 15 mins followed by substrate addition and meas...More data for this Ligand-Target Pair
Affinity DataIC50: 2.59E+5nMAssay Description:In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont...More data for this Ligand-Target Pair
Affinity DataIC50: 9.06E+5nMAssay Description:Inhibition of alpha-glucosidase (unknown origin)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)