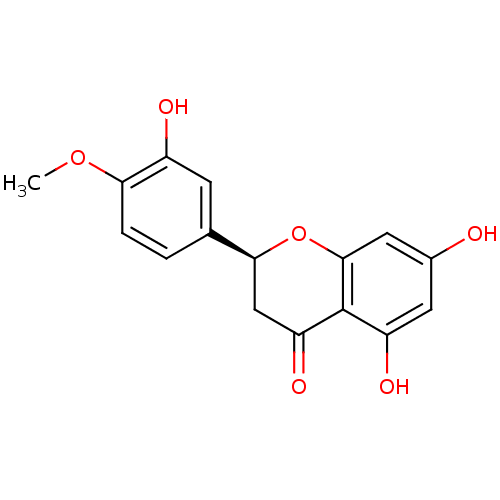
BDBM23418 (2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,4-dihydro-2H-1-benzopyran-4-one::Hesperetin::Hesperitin
SMILES COc1ccc(cc1O)[C@@H]1CC(=O)c2c(O)cc(O)cc2O1
InChI Key InChIKey=AIONOLUJZLIMTK-AWEZNQCLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 19 hits for monomerid = 23418
Found 19 hits for monomerid = 23418
Affinity DataKi: 3.30nM ΔG°: -11.6kcal/moleT: 25°CAssay Description:Inhibition of human recombinant carbonic anhydrase 7 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa...More data for this Ligand-Target Pair
Affinity DataKi: 102nM ΔG°: -9.53kcal/moleT: 25°CAssay Description:Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa...More data for this Ligand-Target Pair
Affinity DataKi: 454nM ΔG°: -8.65kcal/moleT: 25°CAssay Description:Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration ass...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-6.82kcal/moleT: 25°CAssay Description:Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-6.82kcal/moleT: 25°CAssay Description:Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa...More data for this Ligand-Target Pair
Affinity DataIC50: 2.48E+6nMAssay Description:Inhibition of human erythrocyte AChE assessed as acetylthiocholine iodide hydrolysis after 10 mins preincubation by spectrophotometryMore data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Horse)
Graduate School of Gyeongsang National University
Curated by ChEMBL
Graduate School of Gyeongsang National University
Curated by ChEMBL
Affinity DataIC50: 1.77E+6nMAssay Description:Inhibition of equine BChE assessed as butyrylthiocholine iodide hydrolysis after 10 mins preincubation by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 4 secs followed by substrate addition and measured after 30 mins ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.88E+4nMAssay Description:Inhibition of LSD1 (unknown origin) by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.69E+4nMAssay Description:Inhibition of Homo sapiens (human) recombinant GSK3beta after 30 min by Kinase-Glo assayMore data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(SARS-CoV)
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Affinity DataKd: 2.45E+4nMAssay Description:Binding affinity to SARS coronavirus 3C-like protease by SPR analysisMore data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(SARS-CoV)
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Affinity DataIC50: 6.00E+4nMAssay Description:Inhibition of recombinant SARS coronavirus 3C-like protease trans-cleavage activity by ELISAMore data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(SARS-CoV)
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Affinity DataIC50: 8.30E+3nMAssay Description:Inhibition of SARS coronavirus 3C-like protease cis-cleavage activity transfected in african green monkey Vero cells by luciferase reporter gene assa...More data for this Ligand-Target Pair
Affinity DataIC50: 511nMAssay Description:Inhibition of human CYP1B1 by EROD assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.79E+3nMAssay Description:Inhibition of human CYP1A1 by EROD assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.46E+4nMAssay Description:Inhibition of human CYP1A2 by EROD assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+11nMAssay Description:Inhibition of baker's yeast alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate by spectrophotometryMore data for this Ligand-Target Pair
Affinity DatapH: 6.0 T: 22°CAssay Description:The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b...More data for this Ligand-Target Pair
Affinity DataIC50: 5.46E+4nMT: 37°CAssay Description:The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n...More data for this Ligand-Target Pair