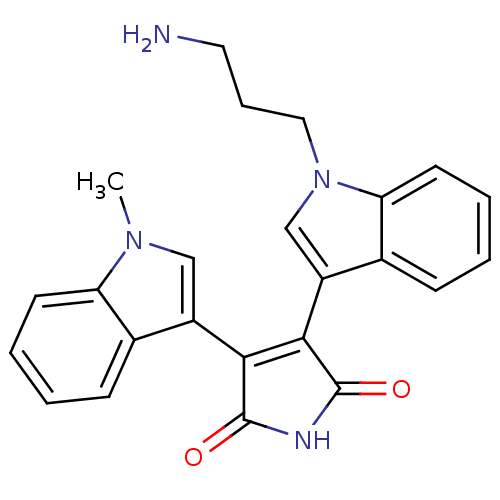
BDBM2695 3-[1-(3-aminopropyl)-1H-indol-3-yl]-4-(1-methyl-1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione::BIM-8::Bisindolylmaleimide VIII::Bisindolylmaleimide deriv. 2b::ROCHE screening, 49::Ro 31-7549
SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(CCCN)c3ccccc23)c2ccccc12
InChI Key InChIKey=UQHKJRCFSLMWIA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 2695
Found 9 hits for monomerid = 2695
Affinity DataIC50: 75nMpH: 7.5 T: 30°CAssay Description:The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his...More data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+3nMpH: 8.5 T: 37°CAssay Description:The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone.More data for this Ligand-Target Pair
Affinity DataIC50: 75nMpH: 7.5 T: 30°CAssay Description:The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his...More data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+3nMpH: 8.5 T: 37°CAssay Description:The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone.More data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase type II subunit delta(Human)
Scios
Curated by ChEMBL
Scios
Curated by ChEMBL
Affinity DataIC50: 310nMAssay Description:Inhibition of CaMK2deltaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.05E+3nMAssay Description:IC50 determinations for TBK1 were performed with the KinEASE-STK assay from Cisbio according to the manufacturer's instructions. A biotinylated s...More data for this Ligand-Target Pair
Affinity DataKd: 12nMAssay Description:Binding affinity to non phosphorylated PIM1More data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase type II subunit delta(Human)
Scios
Curated by ChEMBL
Scios
Curated by ChEMBL
Affinity DataIC50: 310nMAssay Description:Inhibition of CaMK2deltaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMpH: 7.5 T: 30°CAssay Description:In vitro PDK1 assay using purified enzyme, was incubated with substrate PDKtide, and test compounds in the presence of ATP/ [gamma-32P] ATP. 32P inco...More data for this Ligand-Target Pair
Activity Spreadsheet -- ITC Data from BindingDB
 Found 1 hit for monomerid = 2695
Found 1 hit for monomerid = 2695
ITC DataΔG°: -10.3kcal/mole −TΔS°: -0.392kcal/mole ΔH°: -9.88kcal/mole logk: 8.52E+7
pH: 7.5 T: 10.00°C
pH: 7.5 T: 10.00°C
 3D Structure (crystal)
3D Structure (crystal)