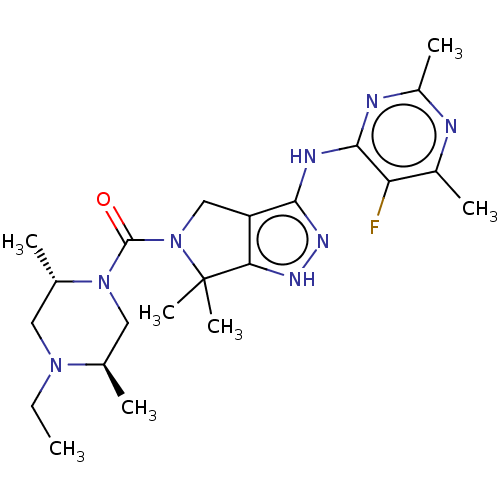
BDBM286254 US11220518, Ex. No. AA3::US11780853, Example AA3::US9518060, Example AA3
SMILES CCN1C[C@H](C)N(C[C@H]1C)C(=O)N1Cc2c(Nc3nc(C)nc(C)c3F)n[nH]c2C1(C)C
InChI Key
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 286254
Found 3 hits for monomerid = 286254
Affinity DataKi: 14.8nMpH: 7.4Assay Description:Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe...More data for this Ligand-Target Pair
Affinity DataKi: 14.8nMAssay Description:Protein Kinase C beta 2 (PKCpII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate peptide...More data for this Ligand-Target Pair
Affinity DataKi: 14.8nMAssay Description:A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a...More data for this Ligand-Target Pair