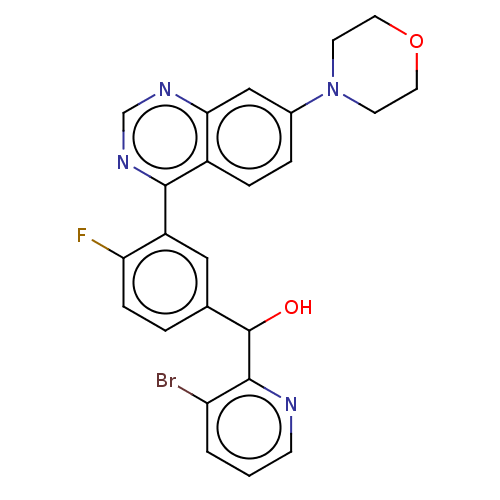
BDBM316056 (3-bromo-pyridin-2- yl)-[4-fluoro-3-(7- morpholin-4-yl- quinazolin-4-yl)- phenyl]methanol1H NMR (400 MHz, DMSO-d6) ppm = 9.09 (s,1H), 8.60 (dd, J = 4.6, 1.4, 1H), 8.08 (dd, J = 8.1,1.5, 1H), 7.65-7.59 (m, 2H), 7.54-7.50 (m, 2H),7.41-7.34 (m, 1H), 7.29 (dd, J = 8.1, 4.6, 1H),7.21-7.18 (m, 1H), 6.20 (d, J = 6.3, 1H), 6.12 (d,J = 6.3, 1H), 3.82-3.74 (m, 4H), 3.49-3.40 (m,4H).::US10172859, Example 478::US11065253, Example 478::US9732094, Example 478
SMILES OC(c1ccc(F)c(c1)-c1ncnc2cc(ccc12)N1CCOCC1)c1ncccc1Br
InChI Key InChIKey=AOTYAPLNLRAVPH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 316056
Found 5 hits for monomerid = 316056
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Patent
US Patent
Merck Patent
US Patent
Affinity DataKi: <1.00E+4nMAssay Description:The patch-clamp measurement was carried out at room temperature in whole-cell configuration on human embryonic kidney cells (HEK293) which have been ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Patent
US Patent
Merck Patent
US Patent
Affinity DataKi: <1.00E+4nMAssay Description:Method for the detection and characterisation of test substances which interfere with the Kv11.1 (hERG) channel: Kv11.1 (hERG, human ether a-go-go re...More data for this Ligand-Target Pair
Affinity DataIC50: <3nMAssay Description:The kinase assay was carried out in streptavidin-coated 348-well microtitre flashplates. To this end, 1.5 μg of DNA-PK/protein complex and 100 n...More data for this Ligand-Target Pair
Affinity DataIC50: <3nMAssay Description:The kinase assay was carried out in streptavidin-coated 348-well microtitre flashplates. To this end, 1.5 μg of DNA-PK/protein complex and 100 n...More data for this Ligand-Target Pair
Affinity DataIC50: <3nMAssay Description:The kinase assay was carried out in streptavidin-coated 348-well microtitre flashplates. To this end, 1.5 ug of DNA-PK/protein complex and 100 ng of ...More data for this Ligand-Target Pair