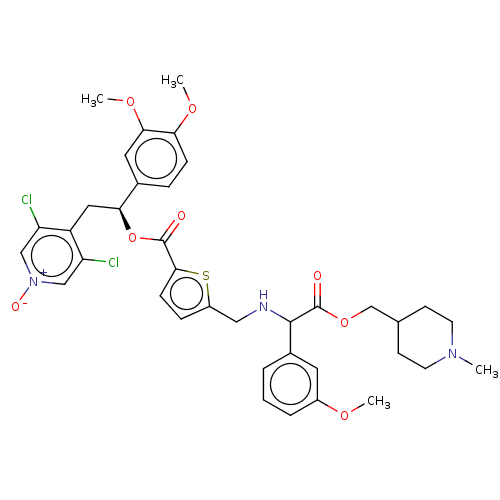
BDBM325715 US9636336, Example 119::US9636336, Example 121::US9636336, Example 122::[(1S)-2-(3,5-dichloro-1-oxido-pyridin-1-ium-4-yl)-1-(3,4-dimethoxyphenyl)ethyl]5-[[[1-(3-methoxyphenyl)-2-[(1-methyl-4-piperidyl)methoxy]-2-oxo-ethyl]amino]methyl]thiophene-2-carboxylate
SMILES COc1cccc(c1)C(NCc1ccc(s1)C(=O)O[C@@H](Cc1c(Cl)c[n+]([O-])cc1Cl)c1ccc(OC)c(OC)c1)C(=O)OCC1CCN(C)CC1
InChI Key InChIKey=QZOOWRHXUJGLAT-GYXLRUHFSA-N
Data 6 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 325715
Found 6 hits for monomerid = 325715
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetIsoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B (PDE4B2)(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
TargetIsoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B (PDE4B2)(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: <1nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
TargetIsoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B (PDE4B2)(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair