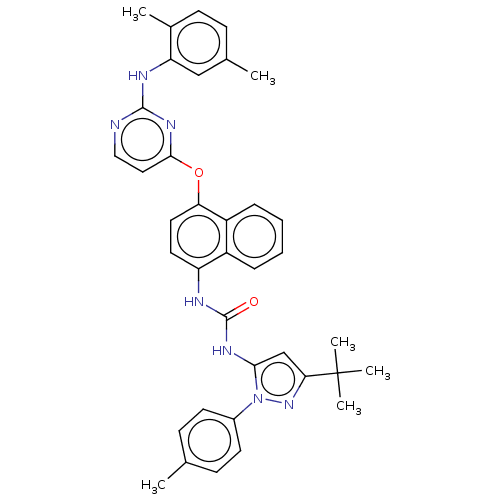
BDBM330268 1-(3-(tert-butyl)-1-(p-tolyl)-1H-pyrazol-5-yl)- 3-(4-((2-((2,5-dimethylphenyl)amino) pyrimidin-4-yl)oxy)naphthalen-1-yl)urea::US10238658, Example 10::US10813932, Example 10::US9724347, Example 10::US9993478, Example 10
SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(Oc2ccnc(Nc3cc(C)ccc3C)n2)c2ccccc12)C(C)(C)C
InChI Key InChIKey=ONVRBAWIVFIJHQ-UHFFFAOYSA-N
Data 12 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 330268
Found 12 hits for monomerid = 330268
Affinity DataIC50: 195nMAssay Description:The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), were evaluated indirectly by determining the lev...More data for this Ligand-Target Pair
Affinity DataIC50: >1.63E+4nMAssay Description:The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), were evaluated in a similar fashion to that descr...More data for this Ligand-Target Pair
Affinity DataIC50: >1.63E+4nMAssay Description:The GSK3-α protein (500 ng/mL, 2.5 μL) was mixed with the test compound (2.5 μL at either 4 μg/mL, 0.4 μg/mL, 0.04 μg/m...More data for this Ligand-Target Pair
Affinity DataIC50: 195nMAssay Description:p38 MAPKα: The enzyme inhibitory activities of compounds disclosed herein were determined by FRET using synthetic peptides labelled with both do...More data for this Ligand-Target Pair
Affinity DataIC50: >1.63E+4nMAssay Description:p38 MAPKγ: The enzyme inhibitory activities of compounds disclosed herein were determined by FRET using synthetic peptides labelled with both do...More data for this Ligand-Target Pair
Affinity DataIC50: >1.63E+4nMAssay Description:GSK 3α: The inhibitory activities of test compounds against the GSK 3α enzyme isoform (Invitrogen), were evaluated by determining the level...More data for this Ligand-Target Pair
Affinity DataIC50: 195nMAssay Description:The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), were evaluated indirectly by determining the lev...More data for this Ligand-Target Pair
Affinity DataIC50: >1.63E+4nMAssay Description:The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), were evaluated in a similar fashion to that descr...More data for this Ligand-Target Pair
Affinity DataIC50: >1.63E+4nMAssay Description:The inhibitory activities of test compounds against the GSK 3α enzyme isoform (Invitrogen), were evaluated by determining the level of activatio...More data for this Ligand-Target Pair
Affinity DataIC50: 195nMAssay Description:p38 MAPKα: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), were evaluated indirectly by det...More data for this Ligand-Target Pair
Affinity DataIC50: >1.63E+4nMAssay Description:p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), were evaluated in a similar fashi...More data for this Ligand-Target Pair
Affinity DataIC50: >1.63E+4nMAssay Description:GSK 3α: The enzyme inhibitory activities of compounds disclosed herein were determined by FRET using synthetic peptides labelled with both donor...More data for this Ligand-Target Pair