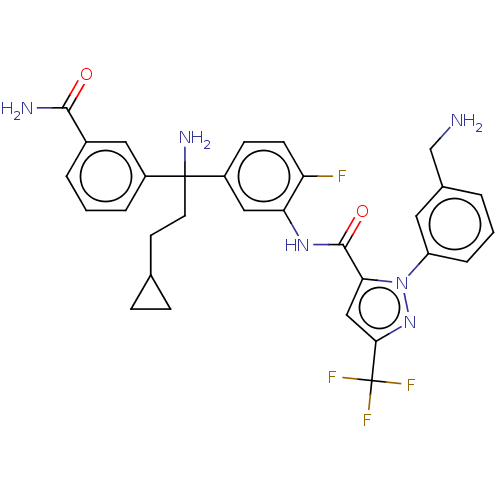
BDBM417014 (+-)-N-(5-(1-amino-1-(3-carbamoylphenyl)-3-cyclopropylpropyl)-2-fluorophenyl)-1-(3-(aminomethyl)phenyl)-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide::US10329260, Compound 225a::US10633345, Compound 225a::US10689346, Compound 225a::US11203574, Compound 225a::US11230530, Compound 225a::US11685721, Compound 225a::US11708332, Compound 225a
SMILES NCc1cccc(c1)-n1nc(cc1C(=O)Nc1cc(ccc1F)C(N)(CCC1CC1)c1cccc(c1)C(N)=O)C(F)(F)F
InChI Key InChIKey=DYGHBBDYRMOWAC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 417014
Found 10 hits for monomerid = 417014
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates. In these experiments, 2...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:Plasma kallikrein activity assay. The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:Plasma kallikrein activity assay. The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic s...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of purified human plasma kallikrein using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of endogenous human plasma kallikrein using Z-Phe-Arg-AMC.HCl as substrate measured after 5 mins by microplate reader analysisMore data for this Ligand-Target Pair