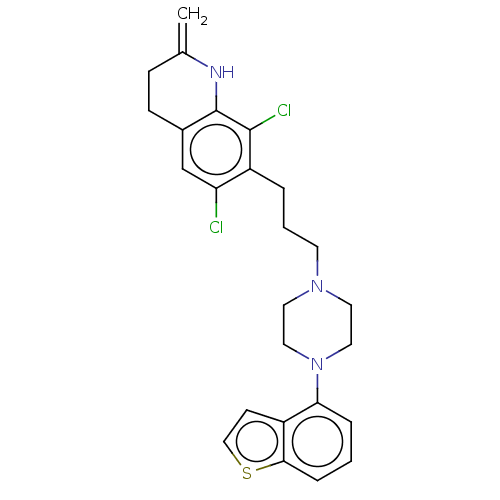
BDBM448341 USRE48059, Compound of Example 163
SMILES Clc1cc2CCC(=C)Nc2c(Cl)c1CCCN1CCN(CC1)c1cccc2sccc12
InChI Key InChIKey=IALCUIBHTWQWLY-UHFFFAOYSA-N
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 448341
Found 2 hits for monomerid = 448341
Affinity DataKi: 2.80nMAssay Description:Dopamine D2: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-raclopride (final concentration 1 to 2 nM)...More data for this Ligand-Target Pair
Affinity DataKi: 7.40nMAssay Description:5-HT2A: The binding assay was performed using 40 μl of the membrane specimen, 20 μl of [3H]-Ketanserin (final concentration 1 to 3 nM), 20 ...More data for this Ligand-Target Pair