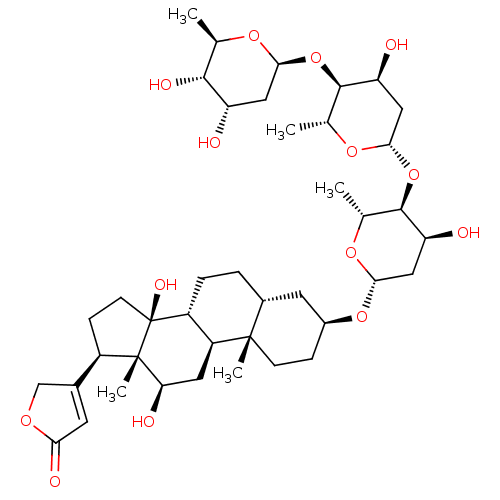
BDBM46355 DIGOXIN::MLS000069819::SMR000059217::US10668094, Compound Digoxin::cid_2724385
SMILES C[C@H]1O[C@H](C[C@H](O)[C@@H]1O)O[C@H]1[C@@H](O)C[C@H](O[C@H]2[C@@H](O)C[C@H](O[C@H]3CC[C@@]4(C)[C@H](CC[C@@H]5[C@@H]4C[C@@H](O)[C@]4(C)[C@H](CC[C@]54O)C4=CC(=O)OC4)C3)O[C@@H]2C)O[C@@H]1C
InChI Key InChIKey=LTMHDMANZUZIPE-PUGKRICDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 35 hits for monomerid = 46355
Found 35 hits for monomerid = 46355
TargetSolute carrier organic anion transporter family member 1A4(Rattus norvegicus)
The University Of Tokyo
Curated by ChEMBL
The University Of Tokyo
Curated by ChEMBL
Affinity DataKi: 37nMAssay Description:TP_TRANSPORTER: inhibition of E217betaG uptake in Oatp2-expressing LLC-PK1 cellsMore data for this Ligand-Target Pair
TargetSodium/potassium-transporting ATPase subunit alpha-2/beta-3(Homo sapiens (Human))
Yeda Research And Development
US Patent
Yeda Research And Development
US Patent
Affinity DataKi: 42.8nMAssay Description:To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo...More data for this Ligand-Target Pair
TargetSodium/potassium-transporting ATPase subunit alpha-2/beta-1(Homo sapiens (Human))
Weizmann Institute Of Science
Weizmann Institute Of Science
Affinity DataKi: 55nMAssay Description:Inhibition of Na,K-ATPase activity of the detergent-soluble α1β1, α2β1, and α3β1 complexes by CGs was done exactly as d...More data for this Ligand-Target Pair
TargetSodium/potassium-transporting ATPase subunit alpha-2/beta-2(Homo sapiens (Human))
Yeda Research And Development
US Patent
Yeda Research And Development
US Patent
Affinity DataKi: 58nMAssay Description:To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo...More data for this Ligand-Target Pair
TargetSodium/potassium-transporting ATPase subunit alpha-2/beta-1(Homo sapiens (Human))
Weizmann Institute Of Science
Weizmann Institute Of Science
Affinity DataKi: 58.7nMAssay Description:To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo...More data for this Ligand-Target Pair
TargetSodium/potassium-transporting ATPase subunit alpha-1/beta-1(Homo sapiens (Human))
Weizmann Institute Of Science
Weizmann Institute Of Science
Affinity DataKi: 189nMAssay Description:Inhibition of Na,K-ATPase activity of the detergent-soluble α1β1, α2β1, and α3β1 complexes by CGs was done exactly as d...More data for this Ligand-Target Pair
TargetSodium/potassium-transporting ATPase subunit alpha-1/beta-1(Homo sapiens (Human))
Weizmann Institute Of Science
Weizmann Institute Of Science
Affinity DataKi: 268nMAssay Description:To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo...More data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1A4(Rattus norvegicus)
The University Of Tokyo
Curated by ChEMBL
The University Of Tokyo
Curated by ChEMBL
Affinity DataKi: 580nMAssay Description:TP_TRANSPORTER: inhibition of Taurocholate uptake in Oatp2-expressing LLC-PK1 cellsMore data for this Ligand-Target Pair
TargetSodium/potassium-transporting ATPase subunit alpha-1(Canis familiaris)
Prassis Istituto Di Ricerche Sigma-Tau
Curated by ChEMBL
Prassis Istituto Di Ricerche Sigma-Tau
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibition of Na+/K+ ATPase from dog kidneyMore data for this Ligand-Target Pair
TargetSodium/potassium-transporting ATPase subunit alpha-1(Canis familiaris)
Prassis Istituto Di Ricerche Sigma-Tau
Curated by ChEMBL
Prassis Istituto Di Ricerche Sigma-Tau
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:In vitro inhibitory concentration against dog kidney Na+,K+-ATPaseMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of P-glycoprotein using calcein-AM assay transfected in porcine PBCECMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of P-glycoprotein, mouse L-mdr1a expressed in LLC-PK1 epithelial cells using calcein-AM polarisation assayMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of P-glycoprotein, human L-MDR1 expressed in LLC-PK1 epithelial cells using calcein-AM polarisation assayMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of human cytochrome P450 3A4More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of P-glycoprotein, mouse L-mdr1b expressed in LLC-PK1 epithelial cells using calcein-AM polarisation assayMore data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
New York University School Of Medicine
Curated by ChEMBL
New York University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Antagonist activity at transactivation domain of RORgammat (unknown origin) expressed in Drosophila Schneider cells co-expressing Gal4-DNA binding do...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:TP_TRANSPORTER: inhibition of Calcein-AM efflux in MDR1-expressing LLC-PK1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.64E+5nMAssay Description:Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair
TargetSodium/potassium-transporting ATPase subunit alpha-4/beta-1(Rattus norvegicus)
University Of Minnesota
Curated by ChEMBL
University Of Minnesota
Curated by ChEMBL
Affinity DataIC50: 5.5nMAssay Description:Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Ten-point DRCs were generated for each drug. Vero cells were seeded at 1.2 × 104 cells per well in DMEM, supplemented with 2% FBS and 1× ...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
New York University School Of Medicine
Curated by ChEMBL
New York University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 8.54E+4nMAssay Description:Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
New York University School Of Medicine
Curated by ChEMBL
New York University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 3.36E+4nMAssay Description:Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
New York University School Of Medicine
Curated by ChEMBL
New York University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 7.01E+3nMAssay Description:Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
New York University School Of Medicine
Curated by ChEMBL
New York University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 2.31E+3nMAssay Description:Orthosteric inverse agonist activity at recombinant human N-terminal His6-tagged RORgammat ligand binding domain (265 to 518 residues) expressed in E...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
New York University School Of Medicine
Curated by ChEMBL
New York University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Orthosteric inverse agonist activity at 2-chloro-5-nitro-N-o-tolylbenzamide-ligated recombinant human N-terminal His6-tagged RORgammat ligand binding...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
New York University School Of Medicine
Curated by ChEMBL
New York University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Orthosteric inverse agonist activity at 2-chloro-N-(2,6-dimethylphenyl)-5-nitrobenzamide-ligated recombinant human N-terminal His6-tagged RORgammat l...More data for this Ligand-Target Pair
TargetSodium/potassium-transporting ATPase subunit alpha-1(Homo sapiens (Human))
Universidade Federal De S£O Jo£O Del Rei
Curated by ChEMBL
Universidade Federal De S£O Jo£O Del Rei
Curated by ChEMBL
Affinity DataIC50: 290nMAssay Description:Inhibition of human kidney Na(+)/K(+) ATPase alpha-1 assessed as amount of Pi release after 1 hr by colorimetric methodMore data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
New York University School Of Medicine
Curated by ChEMBL
New York University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 1.98E+3nMAssay Description:Inhibition of thymus-specific isoform RORgamma (unknown origin) transcriptional activity by luciferase-based cotransfection assayMore data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
New York University School Of Medicine
Curated by ChEMBL
New York University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 4.10E+3nMAssay Description:Displacement of fluorescein-labelled 25-HC from human RoRgamma-LBD by competitive binding assayMore data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
New York University School Of Medicine
Curated by ChEMBL
New York University School Of Medicine
Curated by ChEMBL
Affinity DataKd: 109nMAssay Description:Displacement of [3H]25-hydroxycholesterol from human RORc-LBD expressed in bacterial expression system after 3 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Ku Leuven
Curated by ChEMBL
Ku Leuven
Curated by ChEMBL
Affinity DataIC50: 3.24E+3nMAssay Description:pIC50 values for sodium fluorescein (10 uM) uptake in OATP1B1-transfected CHO cellsMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B3(Homo sapiens (Human))
Ku Leuven
Curated by ChEMBL
Ku Leuven
Curated by ChEMBL
Affinity DataIC50: 1.32E+4nMAssay Description:pIC50 values for sodium fluorescein (10 uM) uptake in OATP1B3-transfected CHO cellsMore data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
New York University School Of Medicine
Curated by ChEMBL
New York University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Orthosteric inverse agonist activity at 2-chloro-5-nitro-N-(2-(trifluoromethyl)phenyl)benzamide-ligated recombinant human N-terminal His6-tagged RORg...More data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3 [702-738,740-752](Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 1.86E+3nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay...More data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 1-alpha/beta(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: >5.57E+4nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)