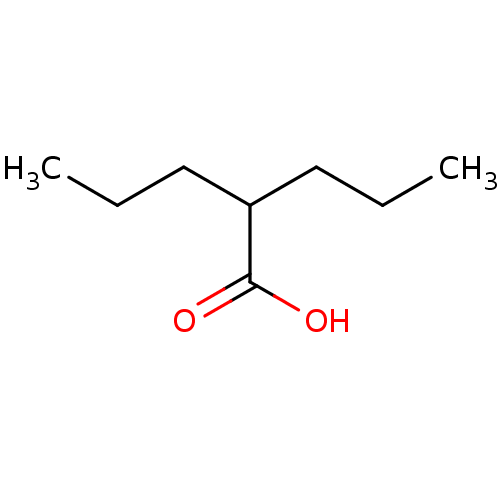
BDBM50003616 2-n-propyl-n-valeric acid::CHEMBL109::DPA::Di-n-propylessigsaeure::VALPROIC ACID::Valproinsaeure::dipropylacetic acid::n-DPA
SMILES CCCC(CCC)C(O)=O
InChI Key InChIKey=NIJJYAXOARWZEE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 46 hits for monomerid = 50003616
Found 46 hits for monomerid = 50003616
TargetSigma non-opioid intracellular receptor 1(Cavia porcellus (Guinea pig))
University Of Catania
Curated by ChEMBL
University Of Catania
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]-pentazocine from sigma1 receptor in guinea pig brain membranes by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3.63E+5nMAssay Description:Inhibition of human CES1More data for this Ligand-Target Pair
Affinity DataKi: 5.64E+5nMAssay Description:Inhibition of HDAC in human Hela cells nuclear extracts by fluorimetric assayMore data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 2B7(Homo sapiens (Human))
The University Of British Columbia
Curated by ChEMBL
The University Of British Columbia
Curated by ChEMBL
Affinity DataKi: 1.60E+6nMAssay Description:Inhibition of AZT glucuronidation by human recombinant UGT2B7More data for this Ligand-Target Pair
Affinity DataKi: 7.90E+6nMAssay Description:Inhibition of human CES2More data for this Ligand-Target Pair
Affinity DataKi: 9.15E+6nMAssay Description:Mechanism based inhibition of human cytochrome P450 2A6 measured by coumarin 7-hydroxylationMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member A1(Rattus norvegicus)
Aristotle University Of Thessaloniki
Curated by ChEMBL
Aristotle University Of Thessaloniki
Curated by ChEMBL
Affinity DataIC50: 5.01E+4nMAssay Description:Inhibition of rat kidney aldehyde reductase at 0.1 mM after 20 mins by spectrometric analysisMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member A1(Rattus norvegicus)
Aristotle University Of Thessaloniki
Curated by ChEMBL
Aristotle University Of Thessaloniki
Curated by ChEMBL
Affinity DataIC50: 5.61E+4nMAssay Description:Inhibition of Fischer-344 rat kidney AK1A1More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member A1(Homo sapiens (Human))
Aristotle University Of Thessaloniki
Curated by ChEMBL
Aristotle University Of Thessaloniki
Curated by ChEMBL
Affinity DataIC50: 5.01E+4nMAssay Description:Inhibition of AK1A1More data for this Ligand-Target Pair
TargetNAD-dependent protein deacylase sirtuin-5, mitochondrial(Homo sapiens (Human))
Technical University Of Denmark
Curated by ChEMBL
Technical University Of Denmark
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of SIRT5More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Aristotle University Of Thessaloniki
Curated by ChEMBL
Aristotle University Of Thessaloniki
Curated by ChEMBL
Affinity DataIC50: 5.61E+4nMAssay Description:Inhibition of Fischer-344 rat kidney ALR1 using D,L-glyceraldehyde as substrate by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+6nMAssay Description:Inhibition of TNF-alpha-induced tissue factor activity in HUVEC preincubated for 4 hrs assessed after 4 hrs of TNFalpha challenge by one stage clotti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+6nMAssay Description:Inhibition of LPS-induced tissue factor activity in HUVEC preincubated for 4 hrs assessed after 4 hrs of LPS challenge by one stage clotting assayMore data for this Ligand-Target Pair
TargetHistone deacetylase 1(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 3.90E+4nMAssay Description:Inhibition of human recombinant HDAC1More data for this Ligand-Target Pair
TargetHistone deacetylase 2(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 6.20E+4nMAssay Description:Inhibition of human recombinant HDAC2More data for this Ligand-Target Pair
TargetHistone deacetylase 3(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1.61E+5nMAssay Description:Inhibition of human recombinant HDAC3More data for this Ligand-Target Pair
TargetHistone deacetylase 8(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1.03E+5nMAssay Description:Inhibition of human recombinant HDAC8More data for this Ligand-Target Pair
TargetHistone deacetylase 4(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: >2.00E+6nMAssay Description:Inhibition of human recombinant HDAC4More data for this Ligand-Target Pair
TargetHistone deacetylase 5(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: >2.00E+6nMAssay Description:Inhibition of human recombinant HDAC5More data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: >2.00E+6nMAssay Description:Inhibition of human recombinant HDAC7More data for this Ligand-Target Pair
TargetHistone deacetylase 9(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: >2.00E+6nMAssay Description:Inhibition of human recombinant HDAC9More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: >2.00E+6nMAssay Description:Inhibition of human recombinant HDAC6More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Aristotle University Of Thessaloniki
Curated by ChEMBL
Aristotle University Of Thessaloniki
Curated by ChEMBL
Affinity DataIC50: 5.61E+4nMAssay Description:Inhibition of Wistar rat kidney ALR1 assessed as reduction in NADPH oxidation using D-glycuronate as substrate by UV-visible spectrophotometric metho...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Aristotle University Of Thessaloniki
Curated by ChEMBL
Aristotle University Of Thessaloniki
Curated by ChEMBL
Affinity DataIC50: 5.61E+4nMAssay Description:Inhibition of ALR1 in Wistar rat kidney assessed as reduction in NADPH consumption preincubated for 1 min followed by substrate addition measured aft...More data for this Ligand-Target Pair
TargetHistone deacetylase 1(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of HADC1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 9.95E+5nMAssay Description:Inhibition of human HDAC in HeLa cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.24E+6nMAssay Description:Inhibition of HDAC in human Hela cells nuclear extracts by fluorimetric assayMore data for this Ligand-Target Pair
TargetHistone deacetylase 1(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 8.42E+4nMAssay Description:Inhibition of human full-length recombinant HDAC1 preincubated for 5 mins followed by HDAC substrate addition and further incubated for 45 mins by fl...More data for this Ligand-Target Pair
TargetHistone deacetylase 2(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1.03E+5nMAssay Description:Inhibition of human full-length recombinant HDAC2 preincubated for 5 mins followed by HDAC substrate addition and further incubated for 45 mins by fl...More data for this Ligand-Target Pair
TargetHistone deacetylase 3(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1.35E+5nMAssay Description:Inhibition of human full-length recombinant HDAC3 preincubated for 5 mins followed by HDAC substrate addition and further incubated for 45 mins by fl...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 2.15E+5nMAssay Description:Inhibition of human full-length recombinant HDAC6 preincubated for 5 mins followed by HDAC substrate addition and further incubated for 45 mins by fl...More data for this Ligand-Target Pair
TargetHistone deacetylase 8(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1.84E+5nMAssay Description:Inhibition of human full-length recombinant C-terminal His-tagged HDAC8 expressed in baculovirus infected Sf9 insect cells preincubated for 5 mins fo...More data for this Ligand-Target Pair
TargetHistone deacetylase 1(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant HDAC1 using fluorogenic substrateMore data for this Ligand-Target Pair
TargetHistone deacetylase 1(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 4.00E+5nMAssay Description:Inhibition of HDAC1 (unknown origin)More data for this Ligand-Target Pair
TargetHistone deacetylase 2(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 7.00E+5nMAssay Description:Inhibition of HDAC2 (unknown origin)More data for this Ligand-Target Pair
TargetHistone deacetylase 3(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 7.00E+5nMAssay Description:Inhibition of HDAC3 (unknown origin)More data for this Ligand-Target Pair
TargetHistone deacetylase 4(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of HDAC4 (unknown origin)More data for this Ligand-Target Pair
TargetHistone deacetylase 5(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of HDAC5 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of human BSEP expressed in plasma membrane vesicles of Sf21 cells assessed as inhibition of ATP-dependent [3H]taurocholate uptakeMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of Sprague-Dawley rat Bsep expressed in plasma membrane vesicles of Sf21 cells assessed as inhibition of ATP-dependent [3H]taurocholate up...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B7(Rattus norvegicus)
Slovak Academy Of Sciences
Curated by ChEMBL
Slovak Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.61E+4nMAssay Description:Inhibition of Wistar rat kidney aldehyde reductase using D-glucuronate as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1 [K65Q](Bos taurus (Cattle))
Quaid-I-Azam University
Curated by ChEMBL
Quaid-I-Azam University
Curated by ChEMBL
Affinity DataIC50: 5.74E+4nMAssay Description:Inhibition of ALR1 in calf kidney using sodium D-glucoronate as substrate treated with compound for 10 mins prior to substrate addition by UV spectro...More data for this Ligand-Target Pair
TargetHistone deacetylase 6(Homo sapiens (Human))
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Broad Institute Of Harvard And Mit
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of HDAC6 (unknown origin)More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member A1(Bos taurus (Cattle))
Comsats Institute Of Information Technology
Comsats Institute Of Information Technology
Affinity DataIC50: 5.74E+4nMpH: 6.2Assay Description:The assay results were obtained at 340 nm and ALR1 inhibitory activity was measured with the absorbance change at this respective wavelength. Each we...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Aristotle University Of Thessaloniki
Curated by ChEMBL
Aristotle University Of Thessaloniki
Curated by ChEMBL
Affinity DataIC50: 5.61E+4nMAssay Description:Inhibition of Fischer-344 rat kidney ALR1 using D-glucuronate as substrate by spectrophotometryMore data for this Ligand-Target Pair