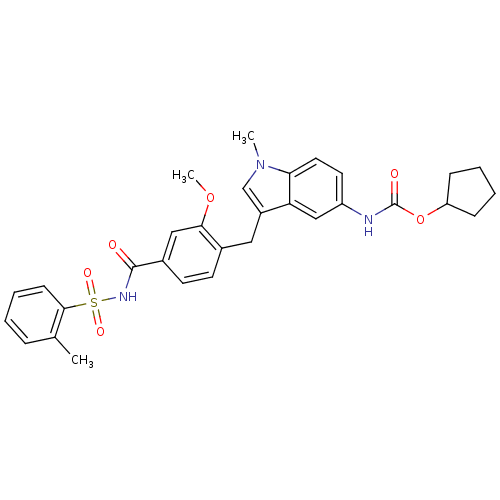
BDBM50009073 4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol-3-ylmethyl)-3-methoxy-N-o-tolylsulfonylbenzamide::CHEMBL603::ZAFIRLUKAST::cyclopentyl 3-(2-methoxy-4-((o-tolylsulfonyl)carbamoyl)benzyl)-1-methylindole-5-carbamate::cyclopentyl 3-[2-methoxy-4-(2-methylphenylsulfonylcarbamoyl)benzyl]-1-methyl-1H-indol-5-ylcarbamate
SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C
InChI Key InChIKey=YEEZWCHGZNKEEK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 56 hits for monomerid = 50009073
Found 56 hits for monomerid = 50009073
TargetCysteinyl leukotriene receptor 1(Homo sapiens (Human))
University Of California Irvine
Curated by PDSP Ki Database
University Of California Irvine
Curated by PDSP Ki Database
Affinity DataKi: 0.300nMAssay Description:Binding affinity of the compound towards Cysteinyl leukotriene D4 receptor (cysLT1) was measured by the displacement of [3H]-LTD4 radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Compound was evaluated for its ability to displace [3H]-LTD4 from Cysteinyl leukotriene D4 receptor in guinea pig lung membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]LTD4 from cysteinyl leukotriene receptor 1 in Hartley guinea pig parenchymal membrane after 30 mins by liquid scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 0.340nMAssay Description:Inhibition constant for displacement of [3H]-LTD4 on guinea pig lung parenchymal membranesMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Antagonism of Cysteinyl leukotriene receptor 1 from guinea pig lung membranesMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Ability to antagonize LTD4 receptors isolated from guinea pig lung membranesMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compound was evaluated in vitro for the binding of cysLT1 receptor to guinea pig lung membranes.More data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Displacement of [3H]-leukotriene D4 (LTD4) from receptor in guinea pig lung membranesMore data for this Ligand-Target Pair
TargetCysteinyl leukotriene receptor 2(Homo sapiens (Human))
University Of California Irvine
Curated by PDSP Ki Database
University Of California Irvine
Curated by PDSP Ki Database
Affinity DataKi: 2.50E+3nMAssay Description:Binding affinity towards cytochrome P450 2C9More data for this Ligand-Target Pair
TargetLeukotriene B4 receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by PDSP Ki Database
Schering-Plough Research Institute
Curated by PDSP Ki Database
TargetLeukotriene B4 receptor 2(Homo sapiens (Human))
University Of Virginia
Curated by PDSP Ki Database
University Of Virginia
Curated by PDSP Ki Database
TargetLeukotriene B4 receptor 2(Homo sapiens (Human))
University Of Virginia
Curated by PDSP Ki Database
University Of Virginia
Curated by PDSP Ki Database
Affinity DataKi: >5.00E+4nMAssay Description:Displacement of [3H]-ligand from platelet activating factor (PAF) receptor in rabbit plateletsMore data for this Ligand-Target Pair
TargetCysteinyl leukotriene receptor 2(Homo sapiens (Human))
University Of California Irvine
Curated by PDSP Ki Database
University Of California Irvine
Curated by PDSP Ki Database
Affinity DataIC50: 5.80E+4nMAssay Description:Antagonist activity against CysLT2 receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization pr...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibitory concentration against muscarinic receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibitory concentration against Beta adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibitory concentration against 5-hydroxytryptamine 2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibitory concentration against Alpha-2 adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibitory concentration against Alpha-1 adrenergic receptorMore data for this Ligand-Target Pair
TargetMultidrug and toxin extrusion protein 2(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 7.60E+3nMAssay Description:Inhibition of human MATE2K-mediated ASP+ uptake expressed in HEK293 cells after 1.5 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetMultidrug and toxin extrusion protein 1(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of human MATE1-mediated ASP+ uptake expressed in HEK293 cells after 1.5 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetSolute carrier family 22 member 2(Homo sapiens (Human))
University Of California
Curated by ChEMBL
University Of California
Curated by ChEMBL
Affinity DataIC50: 9.70E+3nMAssay Description:Inhibition of human OCT2-mediated ASP+ uptake expressed in HEK293 cells after 3 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:In vitro for antagonistic activity against LTD4 receptor in guinea pig ileumMore data for this Ligand-Target Pair
Affinity DataIC50: 1.11E+4nMAssay Description:Inhibition of recombinant human BSEP expressed in baculovirus infected sf9 cell plasma membrane vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair
TargetCysteinyl leukotriene receptor 1(Homo sapiens (Human))
University Of California Irvine
Curated by PDSP Ki Database
University Of California Irvine
Curated by PDSP Ki Database
Affinity DataIC50: 1.80nMAssay Description:Inhibition of cysteinyl leukotriene receptor 1 (unknown origin) expressed in HEK293 cell membranes after 45 mins by scintillation spectrometryMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataEC50: 3.06E+4nMAssay Description:Activation of human PXR expressed in DPX2 cells assessed as CYP3A4 induction after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.21E+4nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.11E+4nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.88E+4nMAssay Description:Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Goethe University Frankfurt
Curated by ChEMBL
Goethe University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition and measured after 30 mins by sp...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Goethe University Frankfurt
Curated by ChEMBL
Goethe University Frankfurt
Curated by ChEMBL
Affinity DataEC50: 2.44E+3nMAssay Description:Partial agonist activity at human PPARgamma LBD expressed HEK293T cells after 12 to 14 hrs by dual-glo luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetSodium/bile acid cotransporter(Homo sapiens (Human))
Southwest Jiaotong University
Curated by ChEMBL
Southwest Jiaotong University
Curated by ChEMBL
Affinity DataIC50: 6.50E+3nMAssay Description:Inhibition of HA-tagged human NTCP expressed in human U2OS cells assessed as reduction in [14C]taurocholate uptake preincubated for 10 mins followed ...More data for this Ligand-Target Pair
TargetCysteinyl leukotriene receptor 1(Homo sapiens (Human))
University Of California Irvine
Curated by PDSP Ki Database
University Of California Irvine
Curated by PDSP Ki Database
Affinity DataIC50: 0.260nMAssay Description:Antagonist activity at human CysLT1 receptorMore data for this Ligand-Target Pair
TargetCysteinyl leukotriene receptor 2(Homo sapiens (Human))
University Of California Irvine
Curated by PDSP Ki Database
University Of California Irvine
Curated by PDSP Ki Database
Affinity DataIC50: >1.00E+3nMAssay Description:Antagonist activity at human CysLT2 receptorMore data for this Ligand-Target Pair
TargetSodium/bile acid cotransporter(Homo sapiens (Human))
Southwest Jiaotong University
Curated by ChEMBL
Southwest Jiaotong University
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibition of preS1-peptide binding to human HA-tagged NTCP in U2OS expresseing NTCP in incubated for 24 hrs using Myrcludex B as substrate by compet...More data for this Ligand-Target Pair
TargetCysteinyl leukotriene receptor 1(Homo sapiens (Human))
University Of California Irvine
Curated by PDSP Ki Database
University Of California Irvine
Curated by PDSP Ki Database
Affinity DataIC50: 14nMAssay Description:Antagonist activity against CysLT1 receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of LTD4-induced calcium mobilization pr...More data for this Ligand-Target Pair
TargetSodium/bile acid cotransporter(Homo sapiens (Human))
Southwest Jiaotong University
Curated by ChEMBL
Southwest Jiaotong University
Curated by ChEMBL
Affinity DataIC50: 6.50E+3nMAssay Description:Inhibition of human NTCP mediated TCA uptake in U2OS expresseing HA-tagged NTCP cells preincubated for 10 mins followed by substrate addition and mea...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibitory concentration against Adenosine A1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibitory concentration against H1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibitory concentration against Dopamine receptor D2More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataEC50: 1.41E+4nMAssay Description:Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysisMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataEC50: 9.50E+3nMAssay Description:Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysisMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataEC50: 710nMAssay Description:Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assayMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Rattus norvegicus)
National Institutes Of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes Of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 5.84E+4nMAssay Description:Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysisMore data for this Ligand-Target Pair
TargetCysteinyl leukotriene receptor 1(Homo sapiens (Human))
University Of California Irvine
Curated by PDSP Ki Database
University Of California Irvine
Curated by PDSP Ki Database
Affinity DataIC50: 1.90nMAssay Description:Antagonist activity at human CysLT1More data for this Ligand-Target Pair
TargetCysteinyl leukotriene receptor 2(Homo sapiens (Human))
University Of California Irvine
Curated by PDSP Ki Database
University Of California Irvine
Curated by PDSP Ki Database
Affinity DataIC50: 7.40E+3nMAssay Description:Antagonist activity at human CysLT2 expressed in HEK293T cells assessed as inhibition of LTC4-induced effect preincubated for 30 mins prior to LTC4 a...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)