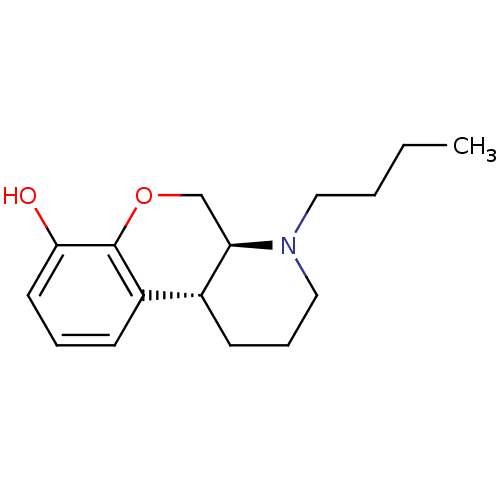
BDBM50016882 1-Butyl-2,3,4,4a,10,10a-hexahydro-1H-9-oxa-1-aza-phenanthren-8-ol::CHEMBL263574
SMILES CCCCN1CCC[C@H]2[C@H]1COc1c(O)cccc21
InChI Key InChIKey=HBUXLNNWKYJTIE-TZMCWYRMSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50016882
Found 4 hits for monomerid = 50016882
Affinity DataIC50: 1.20E+3nMAssay Description:Concentration necessary to achieve half maximal inhibition of [3H]-8-Hydroxy-2-(di-n-propylamino)tetralin binding to 5-hydroxytryptamine 1A receptor ...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Concentration necessary to achieve half maximal inhibition of [3H](+)-23amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene binding to dopamine recepto...More data for this Ligand-Target Pair
Affinity DataIC50: 260nMAssay Description:Concentration necessary to achieve half maximal inhibition of [3H]spiperone binding dopamine receptor at 1 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Concentration necessary to achieve half maximal inhibition of [3H]-clonidine binding to alpha-2 adrenergic receptor at 1 uMMore data for this Ligand-Target Pair