BDBM50022672 CHEMBL3298266
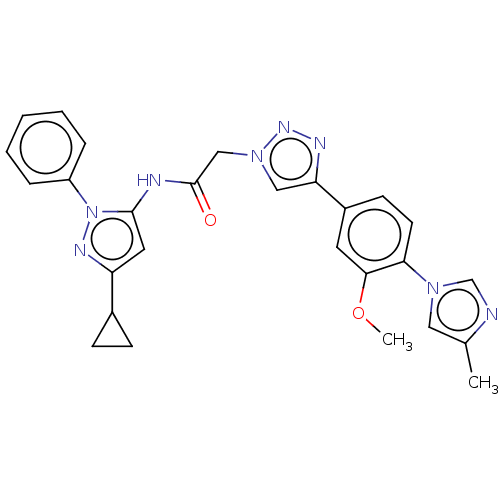
SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(CC(=O)Nc2cc(nn2-c2ccccc2)C2CC2)nn1
InChI Key InChIKey=FQGDIYVVDKJSNC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50022672
Found 8 hits for monomerid = 50022672
TargetHigh affinity nerve growth factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 662nMAssay Description:Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assayMore data for this Ligand-Target Pair
TargetHigh affinity nerve growth factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assayMore data for this Ligand-Target Pair
TargetHigh affinity nerve growth factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKd: 27nMAssay Description:Binding affinity to His-tagged TrkA (unknown origin) after 1 hr by SPR methodMore data for this Ligand-Target Pair
TargetHigh affinity nerve growth factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 287nMAssay Description:Inhibition of juxtamembrane region deficient recombinant human N-terminal His6-tagged/GST-tagged TrkA (498 to 796 residues) expressed in baculovirus ...More data for this Ligand-Target Pair
Affinity DataIC50: 142nMAssay Description:Inhibition of recombinant human TrkC expressed in DHFR deficient CHO cells assessed as inhibition of human neurotrophin-3-induced calcium influx by F...More data for this Ligand-Target Pair
TargetHigh affinity nerve growth factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 85nMAssay Description:Inhibition of recombinant human TrkA expressed in DHFR deficient CHO cells assessed as inhibition of human beta-nerve growth factor-induced calcium i...More data for this Ligand-Target Pair
TargetHigh affinity nerve growth factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 119nMAssay Description:Inhibition of juxtamembrane region containing recombinant human N-terminal His6-tagged/GST-tagged TrkA (473 to 796 residues) expressed in baculovirus...More data for this Ligand-Target Pair
TargetBDNF/NT-3 growth factors receptor(Homo sapiens (Human))
Osaka Prefecture University
Curated by ChEMBL
Osaka Prefecture University
Curated by ChEMBL
Affinity DataIC50: 132nMAssay Description:Inhibition of recombinant human TrkB expressed in DHFR deficient CHO cells assessed as inhibition of human brain-derived neurotrophic factor-induced ...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)