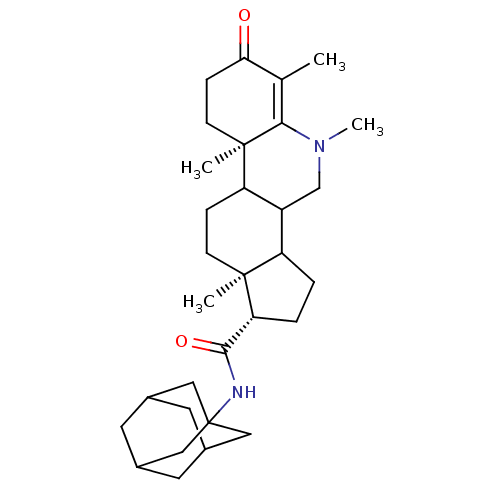
BDBM50039312 (1S,9aR,11aS)-5,6,9a,11a-Tetramethyl-7-oxo-2,3,3a,3b,4,5,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-cyclopenta[i]phenanthridine-1-carboxylic acid adamantan-1-ylamide::CHEMBL307398
SMILES CN1CC2C3CC[C@H](C(=O)NC45CC6CC(CC(C6)C4)C5)[C@@]3(C)CCC2[C@@]2(C)CCC(=O)C(C)=C12
InChI Key InChIKey=DHYYASBDCUAKNN-VCCXQEJISA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50039312
Found 2 hits for monomerid = 50039312
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
Glaxo Inc. Research Institute
Curated by ChEMBL
Glaxo Inc. Research Institute
Curated by ChEMBL
Affinity DataKi: 6.20nMAssay Description:In vitro inhibitory activity against human type 1 5-alpha reductaseMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
Glaxo Inc. Research Institute
Curated by ChEMBL
Glaxo Inc. Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:In vitro inhibitory activity against human type 2 5-alpha reductaseMore data for this Ligand-Target Pair