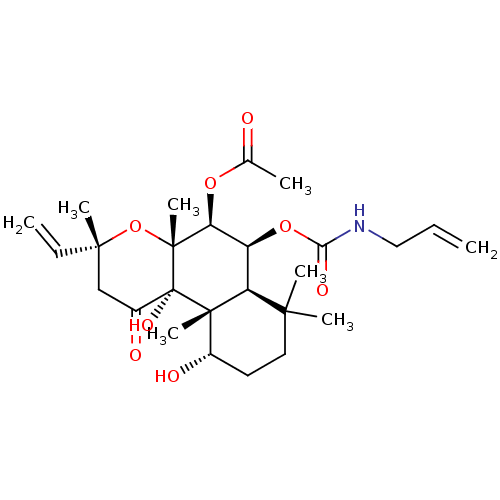
BDBM50052149 Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6-allylcarbamoyloxy-10,10b-dihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-3-vinyl-dodecahydro-benzo[f]chromen-5-yl ester::CHEMBL328358
SMILES CC(=O)O[C@H]1[C@@H](OC(=O)NCC=C)[C@H]2C(C)(C)CC[C@H](O)[C@]2(C)[C@@]2(O)C(=O)C[C@@](C)(O[C@]12C)C=C
InChI Key InChIKey=HHDUOSGLNRNKOV-VYHDIKPLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50052149
Found 3 hits for monomerid = 50052149
Affinity DataEC50: >2.00E+3nMAssay Description:Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1More data for this Ligand-Target Pair
Affinity DataIC50: 76nMAssay Description:Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1More data for this Ligand-Target Pair
Affinity DataEC50: 4.40E+3nMAssay Description:Conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1More data for this Ligand-Target Pair