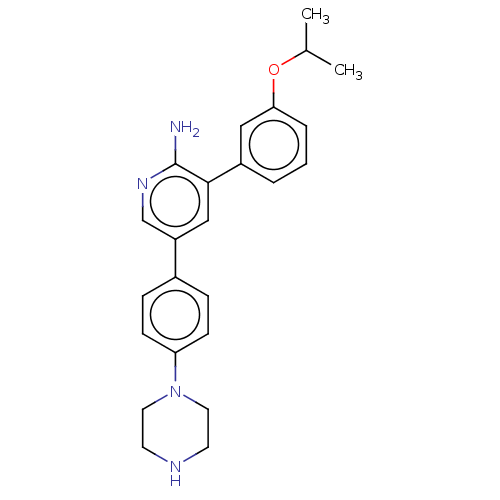
BDBM50056513 CHEMBL3341951
SMILES CC(C)Oc1cccc(c1)-c1cc(cnc1N)-c1ccc(cc1)N1CCNCC1
InChI Key InChIKey=RKDQGZMGXVSYJY-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50056513
Found 4 hits for monomerid = 50056513
TargetActivin receptor type-1(Homo sapiens (Human))
Massachusetts Institute Of Technology
Curated by ChEMBL
Massachusetts Institute Of Technology
Curated by ChEMBL
Affinity DataIC50: 790nMAssay Description:Inhibition of human recombinant human ALK2 kinase after 45 mins by liquid scintillation counting in presence of ATP [gamma-32P]More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of BMP6-induced BMP receptor type 1 ALK2 in mouse C2C12 cells after 30 mins by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Homo sapiens (Human))
Massachusetts Institute Of Technology
Curated by ChEMBL
Massachusetts Institute Of Technology
Curated by ChEMBL
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of TGFbeta1-induced TGFbeta type 1 ALK5 in HEK293T cells after 30 mins by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetTGF-beta receptor type-1(Homo sapiens (Human))
Massachusetts Institute Of Technology
Curated by ChEMBL
Massachusetts Institute Of Technology
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of purified human ALK5 kinase after 45 mins by liquid scintillation counting in presence of ATP [gamma-32P]More data for this Ligand-Target Pair