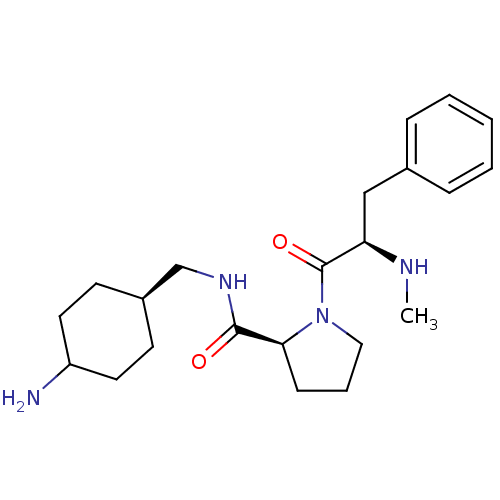
BDBM50056771 (2S)-N-[(4-aminocyclohexyl)methyl]-1-[(2R)-2-(methylamino)-3-phenylpropanoyl]pyrrolidine-2-carboxamide
SMILES CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NC[C@H]1CCC(N)CC1
InChI Key InChIKey=MDSVGJAUFNXYRR-XUBOLADHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50056771
Found 5 hits for monomerid = 50056771
Affinity DataKi: 5nMAssay Description:In vitro inhibitory activity against human thrombin(IIa)More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:In vitro inhibition of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:In vitro inhibitory activity against bovine trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:In vitro inhibition of the compound against bovine trypsin was determinedMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+6nMAssay Description:In vitro inhibitory activity against Coagulation factor XMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)