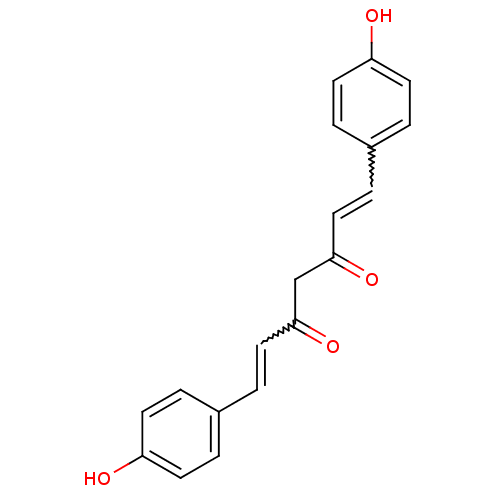
BDBM50059989 (1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-hepta-1,4,6-trien-3-one::(1E,6E)-1,7-Bis-(4-hydroxy-phenyl)-hepta-1,6-diene-3,5-dione::1,7-bis(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione::1,7-bis(4-hydroxyphenyl)-3-hydroxy-1,3,6-heptatrien-5-one::5-Hydroxy-1,7-bis(4-hydroxyphenyl)hepta-1,4,6-trien-3-one::5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-hepta-1,4,6-trien-3-one::CHEMBL105350::CHEMBL131770::bis-demethoxycurcumin::cid_5324473::curcumin III
SMILES Oc1ccc(C=CC(=O)CC(=O)C=Cc2ccc(O)cc2)cc1
InChI Key InChIKey=PREBVFJICNPEKM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 18 hits for monomerid = 50059989
Found 18 hits for monomerid = 50059989
Affinity DataKi: 800nMAssay Description:Inhibition of human MAO-B assessed as inhibition constant using benzylamine as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+4nMAssay Description:Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.82E+4nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase type II subunit alpha(Human)
Rajiv Gandhi Centre For Biotechnology
Curated by ChEMBL
Rajiv Gandhi Centre For Biotechnology
Curated by ChEMBL
Affinity DataIC50: 3.70E+4nMAssay Description:Inhibition of alphaCaMK2 using GST-NR2A as substrate incubated for 1 min prior to substrate addition measured after 1 minMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of GFP-tagged Candida albicans CDR1 expressed in Saccharomyces cerevisiae assessed as inhibition of R6G effluxMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus type 1)
National Cancer Institute-Bethesda
Curated by ChEMBL
National Cancer Institute-Bethesda
Curated by ChEMBL
Affinity DataIC50: 1.20E+5nMAssay Description:Inhibition of 3'- processing activity of HIV-1 integraseMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus type 1)
National Cancer Institute-Bethesda
Curated by ChEMBL
National Cancer Institute-Bethesda
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Compound concentration required to reduce HIV-1 Integrase 3'-processing activity by 50%More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Human)
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
Affinity DataIC50: 3.64E+4nMAssay Description:Anti-oxidant activity in DPPH radicak scavenging assay; n=3-4More data for this Ligand-Target Pair
Affinity DataIC50: 1.83E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's methodMore data for this Ligand-Target Pair
TargetOrphan methyltransferase M.SssI(Spiroplasma monobiae strain MQ-1)
The Ohio State University
Curated by ChEMBL
The Ohio State University
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of Spiroplasma sp. MQ-1 M.SssIMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus type 1)
National Cancer Institute-Bethesda
Curated by ChEMBL
National Cancer Institute-Bethesda
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against HIV-1 Integrase (HIV-1-IN)More data for this Ligand-Target Pair
Affinity DataIC50: 5.10E+3nMAssay Description:Inhibition of AKR1B1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of AKR1B10 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.59E+3nMAssay Description:Inhibition of human MAO-B using benzylamine as substrateMore data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus type 1)
National Cancer Institute-Bethesda
Curated by ChEMBL
National Cancer Institute-Bethesda
Curated by ChEMBL
Affinity DataIC50: 8.00E+4nMAssay Description:Inhibition of strand transfer activity of HIV-1 integraseMore data for this Ligand-Target Pair
TargetSortase family protein(Staphylococcus aureus)
Indian Institute of Technology Delhi
Curated by ChEMBL
Indian Institute of Technology Delhi
Curated by ChEMBL
Affinity DataIC50: 1.04E+5nMAssay Description:Inhibition of beta-hematin formation after 18 hrs by microtiter plate assayMore data for this Ligand-Target Pair
TargetBeta-lactamase(Pseudomonas aeruginosa)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 5.13E+3nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa...More data for this Ligand-Target Pair
TargetToll-like receptor 9(Human)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 9.77E+3nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa...More data for this Ligand-Target Pair