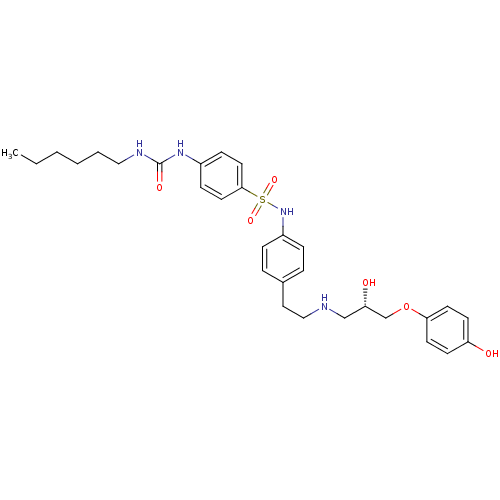
BDBM50070156 (S)-4-(3-hexylureido)-N-(4-(2-(1-hydroxy-2-(4-hydroxyphenoxy)ethylamino)ethyl)phenyl)benzenesulfonamide::(S)-4-(3-hexylureido)-N-(4-(2-(2-hydroxy-3-(4-hydroxyphenoxy)propylamino)ethyl)phenyl)benzenesulfonamide::4-(3-Hexyl-ureido)-N-(4-{2-[(S)-2-hydroxy-3-(4-hydroxy-phenoxy)-propylamino]-ethyl}-phenyl)-benzenesulfonamide::4-(3-Hexyl-ureido)-N-(4-{2-[2-hydroxy-3-(4-hydroxy-phenoxy)-propylamino]-ethyl}-phenyl)-benzenesulfonamide::CHEMBL12998::L-755507
SMILES CCCCCCNC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1ccc(CCNC[C@H](O)COc2ccc(O)cc2)cc1
InChI Key InChIKey=NYYJKMXNVNFOFQ-MHZLTWQESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50070156
Found 14 hits for monomerid = 50070156
Affinity DataKi: 160nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataKi: 570nMAssay Description:Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.430nMAssay Description:Agonism against Beta-3 adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.430nMAssay Description:Evaluated for its agonist activity against human Beta-3 adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.430nMAssay Description:Agonist activity towards human Beta-3 adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:In vitro affinity at Beta-1 adrenergic receptor in the presence of [125I]-iodocyanopindolol.More data for this Ligand-Target Pair
Affinity DataEC50: 1nMAssay Description:In vitro increase in cAMP levels in Chinese hamster ovary cells expressing the human cloned beta 3 Adrenergic receptor.More data for this Ligand-Target Pair
Affinity DataEC50: 20nMAssay Description:Increased cAMP levels of Chinese hamster ovary (CHO) cells expressing human beta 2 adrenergic receptorsMore data for this Ligand-Target Pair
Affinity DataEC50: 330nMAssay Description:In vitro activity to increase cAMP levels in Chinese hamster ovary (CHO) cells expressing the cloned human beta 1 Adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of Tryapanosom cruzi cruzain by Flexstation microplate spectrofluorometryMore data for this Ligand-Target Pair
Affinity DataIC50: >6.00E+4nMAssay Description:Inhibition of Tryapanosom cruzi cruzain by quantitative high throughput screeningMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:In vitro affinity at Beta-2 adrenergic receptor in the presence of [125I]-iodocyanopindolol.More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Beta-2 adrenergic receptor binding affinity in CHO cells expressing cloned human receptor in the presence of 125 I-iodocyanopindololMore data for this Ligand-Target Pair
Affinity DataEC50: 0.430nMAssay Description:Binding affinity of the compound towards human Beta-3 adrenergic receptorMore data for this Ligand-Target Pair