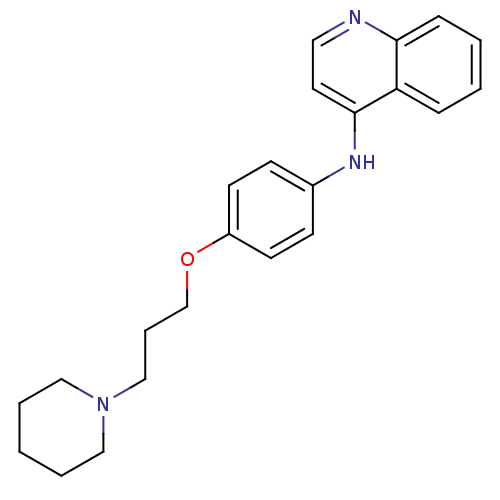
BDBM50110288 CHEMBL15153::N-(4-(3-(piperidin-1-yl)propoxy)phenyl)quinolin-4-amine::[4-(3-Piperidin-1-yl-propoxy)-phenyl]-quinolin-4-yl-amine; Oxalic acid
SMILES C(COc1ccc(Nc2ccnc3ccccc23)cc1)CN1CCCCC1
InChI Key InChIKey=ONLSHDMBVNRMPI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50110288
Found 3 hits for monomerid = 50110288
Affinity DataKi: 0.0910nMAssay Description:Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Displacement of [3H]RAMH from human histamine H3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of rat kidney Histamine N-Methyltransferase (HMT) activity determined by the formation of N-methylhistamineMore data for this Ligand-Target Pair