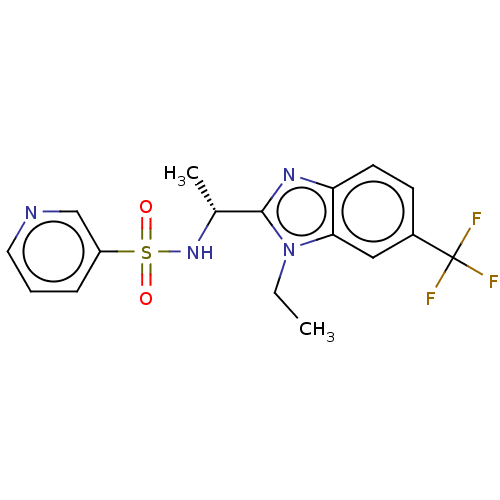
BDBM50113995 CHEMBL3605542
SMILES CCn1c(nc2ccc(cc12)C(F)(F)F)[C@@H](C)NS(=O)(=O)c1cccnc1
InChI Key InChIKey=KJFJNOQRMGDJEA-LLVKDONJSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50113995
Found 3 hits for monomerid = 50113995
Affinity DataIC50: 830nMAssay Description:Inhibition of CYP3A4 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS methodMore data for this Ligand-Target Pair
Affinity DataEC50: 30nMAssay Description:Antagonist activity against human S1P1 receptor expressed in human U2OS cells co-expressing GFP assessed as inhibition of S1P-induced receptor transl...More data for this Ligand-Target Pair
Affinity DataIC50: 830nMAssay Description:Inhibition of CYP3A5 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS methodMore data for this Ligand-Target Pair