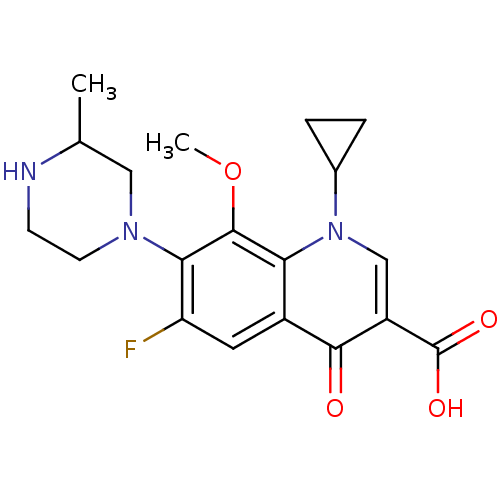
BDBM50117914 1-Cyclopropyl-1,4-dihydro-6-fluoro-8-methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid::1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid::AM 1155::CHEMBL31::GATIFLOXACIN
SMILES COc1c(N2CCNC(C)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1
InChI Key InChIKey=XUBOMFCQGDBHNK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 20 hits for monomerid = 50117914
Found 20 hits for monomerid = 50117914
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tcg Lifesciences
Curated by ChEMBL
Tcg Lifesciences
Curated by ChEMBL
Affinity DataIC50: 1.29E+5nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetDNA gyrase subunit B(Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
Medical School Of Nanjing University
Curated by ChEMBL
Medical School Of Nanjing University
Curated by ChEMBL
Affinity DataIC50: 1.33E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis DNA gyraseMore data for this Ligand-Target Pair
TargetDNA gyrase subunit A/B(Escherichia coli (strain K12))
Achillion Pharmaceuticals
Curated by ChEMBL
Achillion Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibitory activity against wild type Escherichia coli gyraseMore data for this Ligand-Target Pair
TargetDNA topoisomerase 4 subunit A(Staphylococcus aureus)
Achillion Pharmaceuticals
Curated by ChEMBL
Achillion Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4More data for this Ligand-Target Pair
TargetDNA gyrase subunit A/B(Escherichia coli (strain K12))
Achillion Pharmaceuticals
Curated by ChEMBL
Achillion Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibition of Escherichia coli gyraseMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tcg Lifesciences
Curated by ChEMBL
Tcg Lifesciences
Curated by ChEMBL
Affinity DataIC50: 1.29E+5nMAssay Description:Inhibition of human Potassium channel HERG expressed in mammalian cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tcg Lifesciences
Curated by ChEMBL
Tcg Lifesciences
Curated by ChEMBL
Affinity DataIC50: 1.29E+5nMAssay Description:Inhibition of human voltage-gated potassium channel subunit Kv11.1 (ERG K+ channel) in open stateMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tcg Lifesciences
Curated by ChEMBL
Tcg Lifesciences
Curated by ChEMBL
Affinity DataIC50: 1.29E+5nMAssay Description:Inhibition of human ERG in MCF7 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tcg Lifesciences
Curated by ChEMBL
Tcg Lifesciences
Curated by ChEMBL
Affinity DataIC50: 1.29E+5nMAssay Description:Inhibitory concentration against potassium channel HERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tcg Lifesciences
Curated by ChEMBL
Tcg Lifesciences
Curated by ChEMBL
Affinity DataIC50: 1.29E+5nMAssay Description:Inhibition of human ERG expressed in CHO cells by whole cell patch clamp techniqueMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tcg Lifesciences
Curated by ChEMBL
Tcg Lifesciences
Curated by ChEMBL
Affinity DataIC50: 1.30E+5nMAssay Description:K+ channel blocking activity in Chinese hamster ovary cells expressing HERG Kv11.1More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
TargetDNA gyrase subunit B(Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
Medical School Of Nanjing University
Curated by ChEMBL
Medical School Of Nanjing University
Curated by ChEMBL
Affinity DataIC50: 7.99E+3nMAssay Description:Inhibition of Mycobacterium tuberculosis DNA gyrase GyrA/GyrB assessed as reduction of enzyme supercoiling activity using relaxed pBR322 DNA substrat...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tcg Lifesciences
Curated by ChEMBL
Tcg Lifesciences
Curated by ChEMBL
Affinity DataIC50: 1.30E+5nMAssay Description:Inhibition of human ERG current by patch clamp assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8.52E+3nMAssay Description:Inhibition of Staphylococcus aureus wild type DNA gyraseMore data for this Ligand-Target Pair
Affinity DataIC50: 7.64E+5nMAssay Description:Inhibition of Staphylococcus aureus DNA gyrase Ser84Leu mutantMore data for this Ligand-Target Pair
TargetDNA gyrase subunit A(Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
Achillion Pharmaceuticals
Curated by ChEMBL
Achillion Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 9.40E+3nMAssay Description:Inhibition of DNA supercoiling activity of wild type Mycobacterium tuberculosis DNA gyrase AMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)