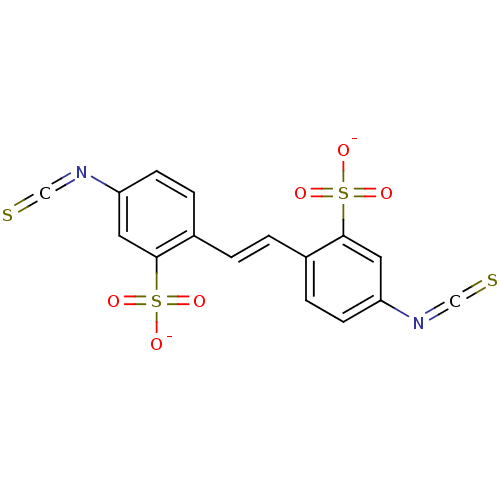
BDBM50118214 CHEMBL133930::DIDS
SMILES [O-]S(=O)(=O)c1cc(ccc1\C=C\c1ccc(cc1S([O-])(=O)=O)N=C=S)N=C=S
InChI Key InChIKey=YSCNMFDFYJUPEF-OWOJBTEDSA-L
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50118214
Found 8 hits for monomerid = 50118214
Affinity DataIC50: >1.00E+5nMAssay Description:The compound was evaluated for antagonist activity against P2X purinoceptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:The compound was evaluated for inhibition of P2X purinoceptor in PC12 bladder cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:The compound was evaluated for antagonist activity against P2X purinoceptor; range 11-54More data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of wild type human RAD51 expressed in Escherichia coli BL21-DE3 using 100 bp ssDNA probe as substrate assessed as prevention of D-loop for...More data for this Ligand-Target Pair
Affinity DataKd: 1.00E+4nMAssay Description:Dissociative constant against P2Y purinoceptor (P2Y) was reported; range 10-214More data for this Ligand-Target Pair
Affinity DataIC50: 750nMAssay Description:Inhibition of RAD51 (unknown origin) assessed as reduction in novel 58/33-dsDNA formation incubated for 1 hr by electrophoresis based DNA strand exch...More data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of RAD51 (unknown origin) using labeled 100-ss DNA as substrate assessed as reduction in DNA D-loop formation by [gamma-32P]ATP based scin...More data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+4nMAssay Description:The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 7 (P2X7)More data for this Ligand-Target Pair