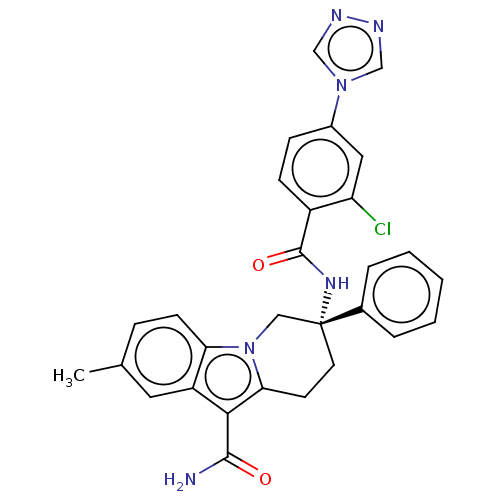
BDBM50126915 CHEMBL3629110::US10351558, Example 138
SMILES Cc1ccc2n3C[C@@](CCc3c(C(N)=O)c2c1)(NC(=O)c1ccc(cc1Cl)-n1cnnc1)c1ccccc1
InChI Key InChIKey=VMFPNMKJFRPSDG-GDLZYMKVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50126915
Found 3 hits for monomerid = 50126915
Affinity DataKi: 2.60nMAssay Description:Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.04E+3nMAssay Description:Inhibition of human F10a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assayMore data for this Ligand-Target Pair