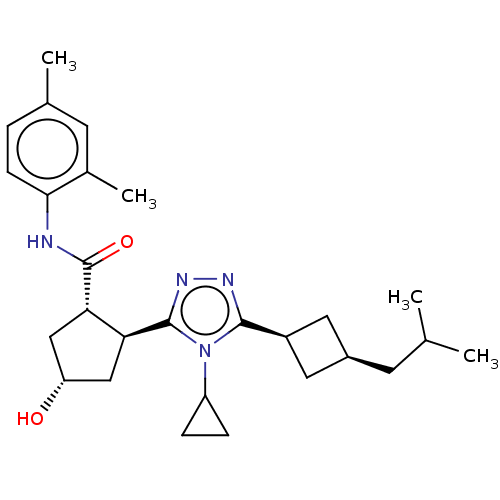
BDBM50153597 CHEMBL3775807
SMILES CC(C)C[C@H]1C[C@H](C1)c1nnc([C@H]2C[C@H](O)C[C@@H]2C(=O)Nc2ccc(C)cc2C)n1C1CC1
InChI Key InChIKey=SEBPCLRPUYJGQT-HUMDQVGNSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50153597
Found 3 hits for monomerid = 50153597
TargetCytochrome P450 3A4(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated with substrate for 5 mins followed by NADPH addition measure...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Mus musculus)
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 38nMAssay Description:Inhibition of mouse RORgamma ligand binding domain (Isoleucine 251 to Lysine 516) expressed in CHOK1 cells incubated for 2 days by Gal4 luciferase re...More data for this Ligand-Target Pair
TargetNuclear receptor ROR-gamma(Homo sapiens (Human))
Central Pharmaceutical Research Institute
Curated by ChEMBL
Central Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 98nMAssay Description:Inhibition of human RORgamma ligand binding domain (Serine 253 to Lysine 518 residues) expressed in CHOK1 cells incubated for 2 days by Gal4 lucifera...More data for this Ligand-Target Pair