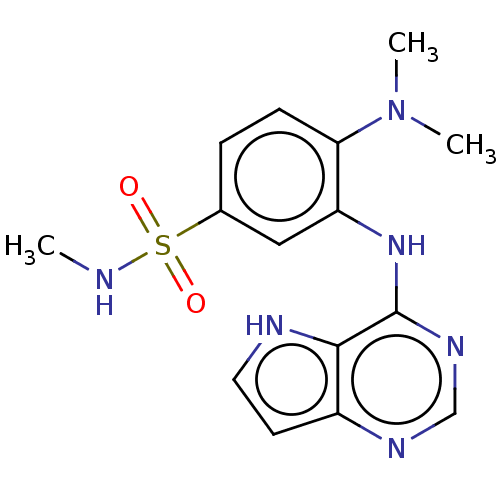
BDBM50178312 CHEMBL3815116
SMILES CNS(=O)(=O)c1ccc(N(C)C)c(Nc2ncnc3cc[nH]c23)c1
InChI Key InChIKey=WAOIOMNKIUTNHS-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50178312
Found 4 hits for monomerid = 50178312
Affinity DataIC50: 500nMAssay Description:Inhibition of human myc-tagged TNNI3K autophosphorylation overexpressed in HEKMSR2 cells incubated for 30 mins by time resolved fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Displacement of 5-({[2-({[3-({4-[(5-hydroxy-2-methylphenyl)amino]-2-pyrimidinyl}amino)phenyl]carbonyl}amino)-ethyl]amino}carbonyl)-2-(6-hydroxy-3-oxo...More data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Inhibition of full-length His6-tagged BRAF V600E mutant (2 to 766 residues) (unknown origin) expressed in baculovirus system by B-Raf accelerated MEK...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Displacement of 5-({[2-({[3-({4-[(5-hydroxy-2-methylphenyl)amino]-2-pyrimidinyl}amino)phenyl]carbonyl}amino)-ethyl]amino}carbonyl)-2-(6-hydroxy-3-oxo...More data for this Ligand-Target Pair