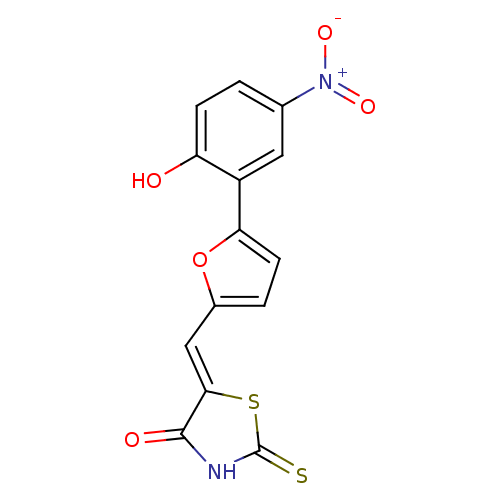
BDBM50189766 5-((5-(2-hydroxy-5-nitrophenyl)furan-2-yl)methylene)-2-thioxothiazolidin-4-one::CHEMBL437688
SMILES Oc1ccc(cc1-c1ccc(\C=C2/SC(=S)NC2=O)o1)[N+]([O-])=O
InChI Key InChIKey=WDMUMCYYKPXDEM-SDQBBNPISA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50189766
Found 3 hits for monomerid = 50189766
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
The University Of Jordan
Curated by ChEMBL
The University Of Jordan
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrateMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
The University Of Jordan
Curated by ChEMBL
The University Of Jordan
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of human PI3KgammaMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of human PI3KalphaMore data for this Ligand-Target Pair