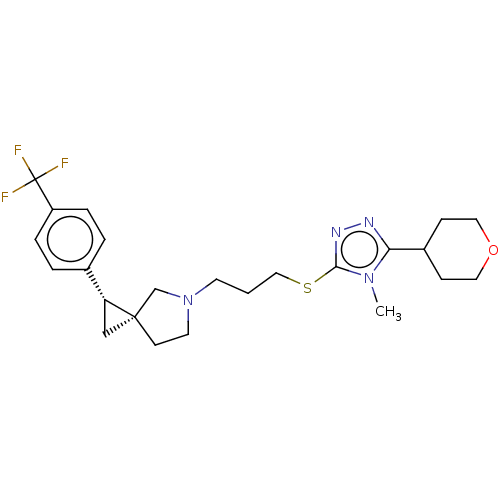
BDBM50192332 CHEMBL3948167::US10239870, Example 70
SMILES Cn1c(SCCCN2CC[C@]3(C[C@@H]3c3ccc(cc3)C(F)(F)F)C2)nnc1C1CCOCC1
InChI Key InChIKey=PPLSEYBUMZWQHS-OFNKIYASSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 50192332
Found 15 hits for monomerid = 50192332
Affinity DataKi: 1.5nMAssay Description:Antagonist activity at human dopamine D3 receptor expressed in CHO cell membranes after 90 mins in presence of quinelorane by [35S]-GTPgammaS binding...More data for this Ligand-Target Pair
Affinity DataKi: 1.66nMAssay Description:[125I]-7OH-PIPAT Binding Assay at rat native D3 receptor on membranes from rat ventral striatum. Homogenates from frozen rat brain ventral striatum (...More data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 251nMAssay Description:Activity at human muscarinic acetylcholine receptor M1 transfected in CHO-K1 cells assessed as intracellular calcium levels in presence of acetylchol...More data for this Ligand-Target Pair
Affinity DataKi: 692nMAssay Description:CHO cells stably expressing human dopamine receptor type 2, long variant (hD2L), coupled to Gα16 protein (CHO-Gα16-hD2L) were seeded into b...More data for this Ligand-Target Pair
Affinity DataKi: 692nMAssay Description:Antagonist activity at human dopamine D2L receptor expressed in CHO cells coexpressing Galpha16 assessed as inhibition of dopamine-induced Ca2+ stimu...More data for this Ligand-Target Pair
Affinity DataKi: 813nMAssay Description:Displacement of [3H]-spiperone from human dopamine D2 receptor expressed in CHO-K1 cell membranes coexpressing Galpha16 after 120 mins by liquid scin...More data for this Ligand-Target Pair
Affinity DataKi: 813nMAssay Description:CHO cells stably expressing human dopamine receptor type 2, long variant (hD2L), coupled to Gα16 protein (CHO-Gα16-hD2L) were re-suspended ...More data for this Ligand-Target Pair
Affinity DataKi: 891nMAssay Description:Activity at human muscarinic acetylcholine receptor M3 transfected in CHO-K1 cells assessed as intracellular calcium levels in presence of acetylchol...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP2D6 expressed in microsomes using MMC as substrate after 10 mins by P450 cypex assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP3A4 expressed in microsomes using DEF as substrate after 10 mins by P450 cypex assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP2C19 expressed in microsomes using BMC as substrate after 10 mins by P450 cypex assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP2C9 expressed in microsomes using FCA as substrate after 10 mins by P450 cypex assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP1A2 expressed in microsomes using ER as substrate after 10 mins by P450 cypex assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Aptuit
Curated by ChEMBL
Aptuit
Curated by ChEMBL
Affinity DataIC50: 2.95E+3nMAssay Description:Inhibition of human ERG transfected in HEK293 cells assessed as reduction in tail current by patch clamp assayMore data for this Ligand-Target Pair