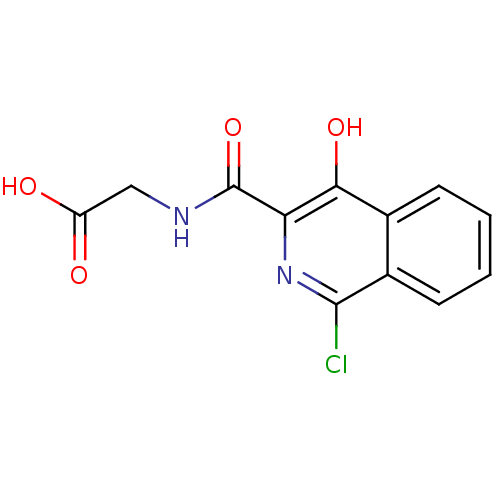
BDBM50193145 2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido)acetic acid::CHEMBL426560::Isoquinoline 3::N-[(1-CHLORO-4-HYDROXYISOQUINOLIN-3-YL)CARBONYL]GLYCINE
SMILES OC(=O)CNC(=O)c1nc(Cl)c2ccccc2c1O
InChI Key InChIKey=OUQVKRKGTAUJQA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 28 hits for monomerid = 50193145
Found 28 hits for monomerid = 50193145
TargetProlyl 4-hydroxylase(Paramecium bursaria Chlorella virus 1)
University Of Oxford
Curated by ChEMBL
University Of Oxford
Curated by ChEMBL
Affinity DataKi: 710nMAssay Description:Inhibition of N-terminal His6-tagged recombinant Paramecium bursaria chlorella virus 1 CPH expressed in Escherichia coli Rosetta 2 (DE3) cells pre-in...More data for this Ligand-Target Pair
TargetAlpha-ketoglutarate-dependent dioxygenase FTO(Homo sapiens (Human))
University Of Oxford
Curated by ChEMBL
University Of Oxford
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of recombinant full length N-terminal hexahistidine-tagged human FTO demethylation activity expressed in Escherichia coli BL21 (DE3) incub...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.5Assay Description:Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.5Assay Description:Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.5Assay Description:Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+5nMpH: 7.5Assay Description:Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.5Assay Description:Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o...More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of human BBOX pre-incubated for 10 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+4nMAssay Description:Inhibition of human BBOX pre-incubated for 1 min using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assayMore data for this Ligand-Target Pair
Affinity DataKd: 1.22E+5nMAssay Description:Binding affinity to human BBOX by tryptophan fluorescence quenching binding assayMore data for this Ligand-Target Pair
Affinity DataKd: 1.30E+4nMAssay Description:Binding affinity to human BBOX in presence of Fe(II) by tryptophan fluorescence quenching binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of human BBOX pre-incubated for 20 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of recombinant PHD2 (unknown origin) catalytic domain (181 to 426 residues) expressed in Escherichia coli BL21 (DE3) cells using boitinyla...More data for this Ligand-Target Pair
Affinity DataIC50: 424nMAssay Description:Displacement of FITC-HIF-1alpha (556 to 574 residues) from PHD2 (181 to 426 residues) (unknown origin) after 60 mins by fluorescence polarization ass...More data for this Ligand-Target Pair
Affinity DataIC50: 73nMAssay Description:Inhibition of human PHD2 at 293K temperature by solvent relaxation techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human EGLN1More data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of PHD2 (unknown origin) after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetAlpha-ketoglutarate-dependent dioxygenase FTO(Homo sapiens (Human))
University Of Oxford
Curated by ChEMBL
University Of Oxford
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of human hexahistidine-tagged full-length FTO expressed in Escherichia coli BL21 (DE3) using 3-methylthymidine as substrate assessed as in...More data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of human PHD2 catalytic domain (181 to 426) Mn2 expressed in Escherichia coli by NMR spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataKd: 80nMAssay Description:Binding affinity to human PHD2 catalytic domain (181 to 426) Mn2 expressed in Escherichia coli by NMR spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+3nMAssay Description:Inhibition of human HIF-PHD2 assessed as reduction in HIF1-alpha binding to VBC complex using biotin-labeled HIF1-alpha peptide as substrate preincub...More data for this Ligand-Target Pair
Affinity DataEC50: 7.90E+4nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of PHD2 (181 to 426 residues) (unknown origin) using biotinylated CODD peptide as substrate preincubated for 15 mins followed by substrate...More data for this Ligand-Target Pair
TargetProlyl 4-hydroxylase(Paramecium bursaria Chlorella virus 1)
University Of Oxford
Curated by ChEMBL
University Of Oxford
Curated by ChEMBL
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of N-terminal His6-tagged recombinant Paramecium bursaria chlorella virus 1 CPH expressed in Escherichia coli Rosetta 2 (DE3) cells pre-in...More data for this Ligand-Target Pair
Affinity DataIC50: 640nMAssay Description:Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.5Assay Description:Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.5Assay Description:Inhibition assays were carried out in 384-well white ProxiPlates(PerkinElmer) in 10 μL of reaction volume. Standard reaction mixturesconsisted o...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)