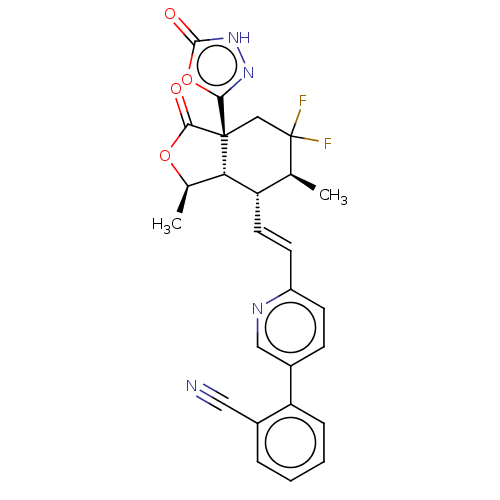
BDBM50197613 CHEMBL3940415::US10322140, Example 1
SMILES [H][C@@]12[C@@H](C)OC(=O)[C@@]1(CC(F)(F)[C@@H](C)[C@@H]2\C=C\c1ccc(cn1)-c1ccccc1C#N)c1n[nH]c(=O)o1
InChI Key InChIKey=VUPRNTCJYFKOPE-RQSFRHHQSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50197613
Found 3 hits for monomerid = 50197613
Affinity DataIC50: 2.18E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate after 10 mins in presence of NADP by rapidfire/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.18E+4nMAssay Description:Compound dilutions and assay-ready plates were prepared on a TTP Labtech mosquito HTS. Assay conduction was fully automated on a customized Screening...More data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Therachem Research Medilab (India)
Curated by ChEMBL
Therachem Research Medilab (India)
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Antagonist activity at PAR-1 in HEK293 cells incubated for 30 mins followed by Ala-parafluoroPhe-Arg-Cha-Cit-Try-NH2 substrate addition by calcium-5 ...More data for this Ligand-Target Pair