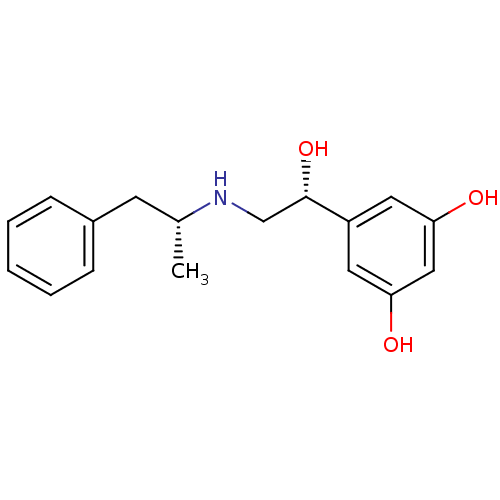
BDBM50213108 (R,R)-(-)-5-{2-[2-(4-aminophenyl)-1-methylethylamino]-1-hydroxyethyl}-1,3-benzenediol::CHEMBL389629::US10617654, Compound (R,R)-3::US9492405, (R,R)-3::US9492405, 9
SMILES C[C@H](Cc1ccccc1)NC[C@H](O)c1cc(O)cc(O)c1
InChI Key InChIKey=GGCAKMLDJKMBIK-PXAZEXFGSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50213108
Found 8 hits for monomerid = 50213108
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
The United States Of America, As Represented By The Secretary, Department Of Health And Human Services
US Patent
The United States Of America, As Represented By The Secretary, Department Of Health And Human Services
US Patent
Affinity DataKi: 2.93E+3nM ΔG°: -7.01kcal/molepH: 7.8 T: 2°CAssay Description:Beta1-AR binding was done on rat cortical membrane following a previously described procedure (Beer et al., Biochem. Pharmacol. 37: 1145-1151, 1988)....More data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
The United States Of America, As Represented By The Secretary, Department Of Health And Human Services
US Patent
The United States Of America, As Represented By The Secretary, Department Of Health And Human Services
US Patent
Affinity DataKi: 3.00E+3nMAssay Description:Displacement of [3H]CGP12177 from human beta-2 adrenergic receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetBeta-1 adrenergic receptor(Rattus norvegicus (Rat))
Medical University Of Lublin
Curated by ChEMBL
Medical University Of Lublin
Curated by ChEMBL
Affinity DataKi: 2.49E+4nMAssay Description:Displacement of [3H]CGP-12177 from beta-1 adrenergic receptor in Sprague-Dawley rat cortical membraneMore data for this Ligand-Target Pair
TargetBeta-1 adrenergic receptor(Rattus norvegicus (Rat))
Medical University Of Lublin
Curated by ChEMBL
Medical University Of Lublin
Curated by ChEMBL
Affinity DataKi: 2.50E+4nM ΔG°: -5.83kcal/molepH: 7.8 T: 2°CAssay Description:Beta1-AR binding was done on rat cortical membrane following a previously described procedure (Beer et al., Biochem. Pharmacol. 37: 1145-1151, 1988)....More data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
The United States Of America, As Represented By The Secretary, Department Of Health And Human Services
US Patent
The United States Of America, As Represented By The Secretary, Department Of Health And Human Services
US Patent
Affinity DataKi: 2.93E+6nMAssay Description:HEK 293 cells stability transfected with cDNA encoding human β2-AR (provided by Dr. Brian Kobilka, Stanford Medical Center, Palo Alto, Calif.) w...More data for this Ligand-Target Pair
TargetBeta-1 adrenergic receptor(Mus musculus)
The Usa, As Represented By The Secretary, Department Of Health And Human Services
US Patent
The Usa, As Represented By The Secretary, Department Of Health And Human Services
US Patent
Affinity DataKi: 2.50E+7nMAssay Description:β1-AR binding was done on rat cortical membrane following a previously described procedure (Beer et al., Biochem. Pharmacol. 37: 1145-1151, 1988...More data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
The United States Of America, As Represented By The Secretary, Department Of Health And Human Services
US Patent
The United States Of America, As Represented By The Secretary, Department Of Health And Human Services
US Patent
Affinity DataKd: 2.95E+3nMpH: 7.7Assay Description:HEK 293 cells stability transfected with cDNA encoding human beta2-AR (provided by Dr. Brian Kobilka, Stanford Medical Center, Palo Alto, Calif.) wer...More data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
The United States Of America, As Represented By The Secretary, Department Of Health And Human Services
US Patent
The United States Of America, As Represented By The Secretary, Department Of Health And Human Services
US Patent
Affinity DataKd: 2.95E+3nMAssay Description:HEK 293 cells stability transfected with cDNA encoding human β2-AR (provided by Dr. Brian Kobilka, Stanford Medical Center, Palo Alto, Calif.) w...More data for this Ligand-Target Pair