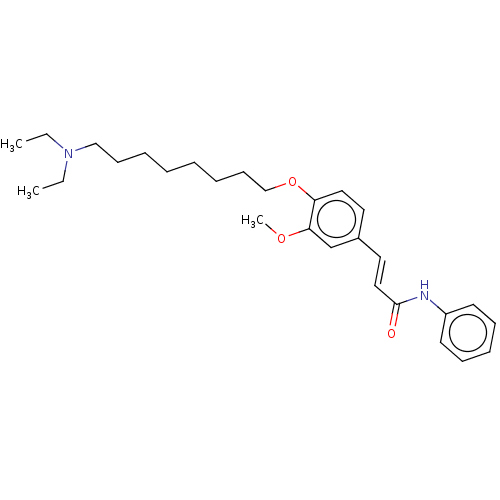
BDBM50232310 CHEMBL4105158
SMILES CCN(CC)CCCCCCCCOc1ccc(\C=C\C(=O)Nc2ccccc2)cc1OC
InChI Key InChIKey=CEMCHKPKVITYSX-CZIZESTLSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50232310
Found 2 hits for monomerid = 50232310
Affinity DataIC50: 1.36E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.98E+3nMAssay Description:Inhibition of butyrylcholinesterase (unknown origin) using butyrylthiocholine iodide as substrate after 25 mins by Ellmann methodMore data for this Ligand-Target Pair