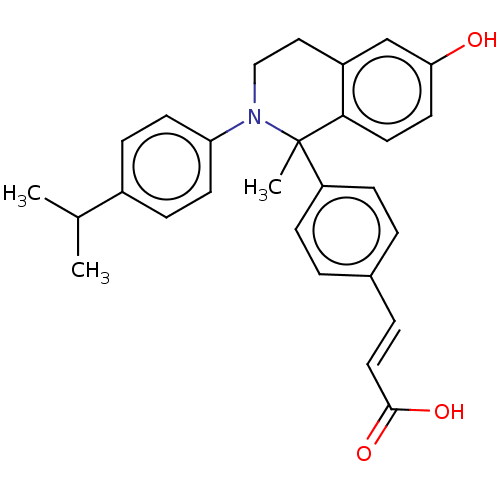
BDBM50237302 CHEMBL4101697
SMILES CC(C)c1ccc(cc1)N1CCc2cc(O)ccc2C1(C)c1ccc(\C=C\C(O)=O)cc1
InChI Key InChIKey=HKXVKUOGCLJVEZ-GIDUJCDVSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50237302
Found 4 hits for monomerid = 50237302
TargetEstrogen receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Antagonist activity at ERalpha in human MCF7 cells assessed as inhibition of estrogen-induced transcription preincubated overnight followed by estrog...More data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by Western blot analysisMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Induction of ERalpha degradation in human MCF7 cells assessed as inhibition of insulin-mediated cell proliferation after 6 days by Hoechst 33258 dye-...More data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Induction of ERalpha degradation in human MCF7 cells after 18 hrs by Western blot analysisMore data for this Ligand-Target Pair