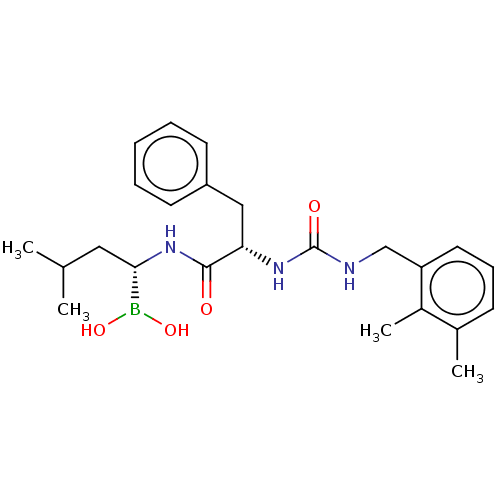
BDBM50259642 CHEMBL4077037
SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(C)c1C)B(O)O
InChI Key InChIKey=ZWXBHHXHLYJPNQ-VXKWHMMOSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50259642
Found 3 hits for monomerid = 50259642
Affinity DataIC50: 4nMAssay Description:Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ...More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of caspase-like activity of 20S proteasome in human HL60 cells using Z-nLPnLDaminoluciferin as substrate after 2 hrs by fluorescence analy...More data for this Ligand-Target Pair
Affinity DataIC50: 340nMAssay Description:Inhibition of trypsin-like activity of 20S proteasome in human HL60 cells using Z-LRRaminoluciferin as substrate after 2 hrs by fluorescence analysisMore data for this Ligand-Target Pair