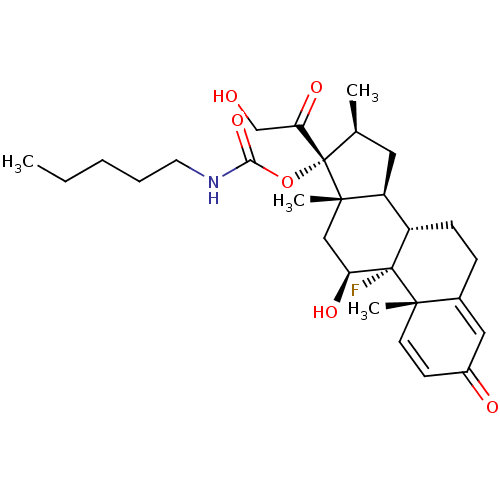
BDBM50272400 (8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl pentylcarbamate::CHEMBL499630
SMILES CCCCCNC(=O)O[C@@]1([C@@H](C)C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)CO
InChI Key InChIKey=PXEYRAVUWVPDKI-DGAWKMIRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50272400
Found 3 hits for monomerid = 50272400
Affinity DataEC50: 18nMAssay Description:Inhibition of glucocorticoid-mediated human MMP1 expression in PMA stimulated A549 cells assessed as MMP1 protein level by ELISAMore data for this Ligand-Target Pair
Affinity DataEC50: 117nMAssay Description:Transactivation activity of tyrosine amino transferase in rat H4 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair