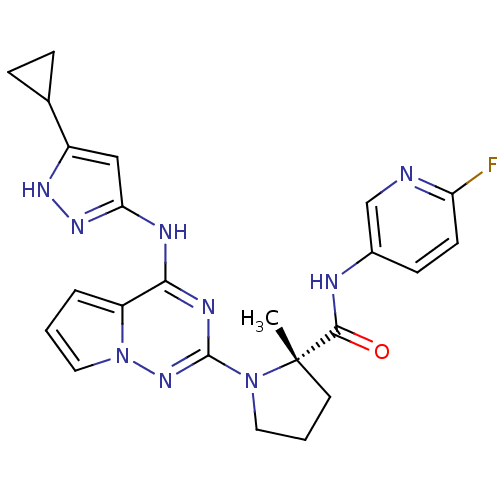
BDBM50299148 (S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrrolo[1,2-f][1,2,4]triazin-2-yl)-N-(6-fluoropyridin-3-yl)-2-methylpyrrolidine-2-carboxamide::1-{4-[(3-cyclopropyl-1H-pyrazol-5-yl)amino]pyrrolo[2,1-f][1,2,4]triazin-2-yl}-N-(6-fluoropyridin-3-yl)-2-methyl-L-prolinamide::BMS-754807::CHEMBL575448
SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1
InChI Key InChIKey=LQVXSNNAFNGRAH-QHCPKHFHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 22 hits for monomerid = 50299148
Found 22 hits for monomerid = 50299148
Affinity DataKd: 525nMAssay Description:Kinobeads competition assays were performed in 96-well format as previously described using mixed protein lysates of four cancer cell lines (K-562, M...More data for this Ligand-Target Pair
Affinity DataIC50: 3.16E+3nMAssay Description:Inhibition of LMTK3 (unknown origin)More data for this Ligand-Target Pair
TargetInsulin-like growth factor 1 receptor(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of IGF1R in IGF1R-SAL cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP3A4 using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of CYP3A4 using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of Akt phosphorylation in mouse Sal cells by western blottingMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of IGF1R phosphorylation in mouse Sal cells by western blottingMore data for this Ligand-Target Pair
TargetInsulin-like growth factor 1 receptor(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of IGF1R after 60 mins by fluorescence electrophoresisMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2(Human)
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 1.04E+3nMAssay Description:Inhibition of CDK2/Cyclin E after 60 mins by fluorescence electrophoresisMore data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Human)
Rheinische Friedrich-Wilhelms-University of Bonn
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-University of Bonn
Curated by ChEMBL
Affinity DataIC50: 1.02E+4nMAssay Description:Inhibition of ABCG2 (unknown origin) expressed in MDCK2 cells co-expressing BCRP (unknown origin) assessed as effect on pheophorbide A accumulation p...More data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Human)
Rheinische Friedrich-Wilhelms-University of Bonn
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-University of Bonn
Curated by ChEMBL
Affinity DataIC50: 1.77E+4nMAssay Description:Inhibition of ABCB1 in human A2780/ADR cells preincubated for 30 mins followed by calcein AM addition and measured at 60 secs time interval by fluore...More data for this Ligand-Target Pair
TargetMultidrug resistance-associated protein 1(Human)
Rheinische Friedrich-Wilhelms-University of Bonn
Curated by ChEMBL
Rheinische Friedrich-Wilhelms-University of Bonn
Curated by ChEMBL
Affinity DataIC50: 3.63E+4nMAssay Description:Inhibition of ABCC1 in human H69AR cells preincubated for 30 mins followed by calcein-AM addition measured at 60 secs time interval by fluorescence a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of IR (unknown origin)More data for this Ligand-Target Pair
TargetInsulin-like growth factor 1 receptor(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of IGF-1R (unknown origin)More data for this Ligand-Target Pair
TargetHigh affinity nerve growth factor receptor(Human)
University of Arkansas For Medical Sciences
Curated by ChEMBL
University of Arkansas For Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of TRKA (unknown origin)More data for this Ligand-Target Pair
TargetBDNF/NT-3 growth factors receptor(Human)
University of Arkansas For Medical Sciences
Curated by ChEMBL
University of Arkansas For Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of TRKB (unknown origin)More data for this Ligand-Target Pair
TargetInsulin-like growth factor 1 receptor(Human)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL