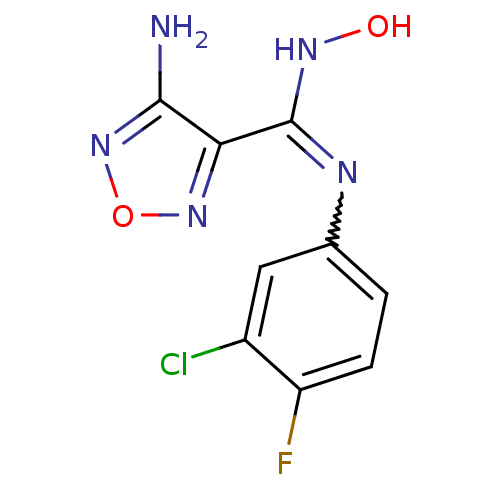
BDBM50300305 4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2,5-oxadiazole-3-carboximidamide::CHEMBL584991::US10669273, Compound INCB024360
SMILES Nc1nonc1C(NO)=Nc1ccc(F)c(Cl)c1
InChI Key InChIKey=HGXSLPIXNPASGZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 19 hits for monomerid = 50300305
Found 19 hits for monomerid = 50300305
Affinity DataKi: 22nMAssay Description:Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft...More data for this Ligand-Target Pair
Affinity DataKi: 3.40E+4nMAssay Description:Competitive inhibition of IDO1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of N-terminal 6xHis-tagged human TDO expressed in Escherichia cli Rosetta (DE3) pLysS using L-tryptophan as substrate preincubated for 1 h...More data for this Ligand-Target Pair
Affinity DataEC50: 59nMAssay Description:Inhibition of IDO1 in IFNgamma-induced human MDA-MB-231 cells using tryptophan as substrate preincubated for 4 hrs followed by substrate addition for...More data for this Ligand-Target Pair
Affinity DataIC50: 67nMAssay Description:Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 46nMAssay Description:Inhibition of indoleamine 2,3-dioxygenase in mouse B16 cells assessed as kynurenine formation by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of tryptophan 2,3-dioxygenaseMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of tryptophan 2,3-dioxygenase by cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of indoleamine 2,3-dioxygenase in mouse B16 cells assessed as kynurenine formation by adjusted cell-based spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysisMore data for this Ligand-Target Pair
TargetTryptophan 2,3-dioxygenase(Mouse)
Ludwig Center For Cancer Research of The University of Lausanne
Curated by ChEMBL
Ludwig Center For Cancer Research of The University of Lausanne
Curated by ChEMBL
Affinity DataIC50: 6.00E+4nMAssay Description:Inhibition of mouse TDO in P815 clone 12 cells by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+4nMAssay Description:Inhibition of human TDO transfected in mouse P815B clone 19 cells by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 75nMAssay Description:Inhibition of human N-terminal His-tagged IDO1 expressed in Escherichia coli using D-Trp as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 91nMAssay Description:Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft...More data for this Ligand-Target Pair
Affinity DataIC50: 78nMAssay Description:Inhibition of N-terminus 6xHis-tagged human IDO1 expressed in Escherichia coli M15 using L-tryptophan as substrate preincubated for 1 hr measured aft...More data for this Ligand-Target Pair