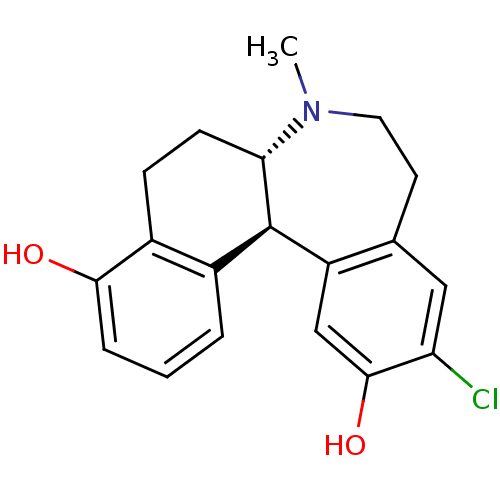
BDBM50306435 (6aS,13bS)-11-chloro-7-methyl-6,6a,7,8,9,13b-hexahydro-5H-benzo[d]naphtho[2,1-b]azepine-4,12-diol::CHEMBL604314
SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1c(O)cccc21
InChI Key InChIKey=RANYBZPZDFYJIV-QFBILLFUSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50306435
Found 4 hits for monomerid = 50306435
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Displacement of radioligand from dopamine D5 receptor expressed in mouse LTK cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 440nMAssay Description:Displacement of [3H]methylspiperon from dopamine D2 receptor expressed in mouse LTK cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3.99E+3nMAssay Description:Displacement of radioligand from dopamine D4 receptor expressed in mouse LTK cells by scintillation countingMore data for this Ligand-Target Pair