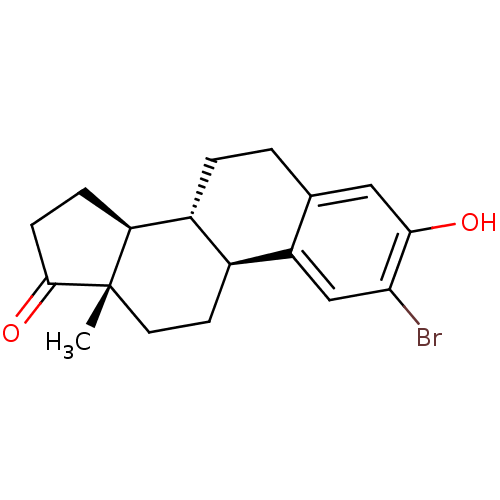
BDBM50311659 (8R,9S,13S,14S)-2-Bromo-3-hydroxy-13-methyl-6,7,8,9,11,12,13,14,15,16-decahydro-cyclopenta[a]phenanthren-17-one(8R,9S,13S,14S)-2-Bromo-3-hydroxy-13-methyl-6,7,8,9,11,12,13,14,15,16-decahydro-cyclopenta[a]phenanthren-17-one::(8R,9S,13S,14S)-2-bromo-3-hydroxy-13-methyl-7,8,9,11,12,13,15,16-octahydro-6H-cyclopenta[a]phenanthren-17(14H)-one::CHEMBL189051
SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(Br)cc34)[C@@H]1CCC2=O
InChI Key InChIKey=JVITVXNLPKMREE-JPVZDGGYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50311659
Found 3 hits for monomerid = 50311659
Affinity DataKi: 100nMAssay Description:Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]ADMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
Institute Of Experimental Genetics
Curated by ChEMBL
Institute Of Experimental Genetics
Curated by ChEMBL
Affinity DataIC50: 233nMAssay Description:Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human steroid sulfatase using 4-methylumbelliferyl sulfate substrate after 10 mins by fluorescence assayMore data for this Ligand-Target Pair