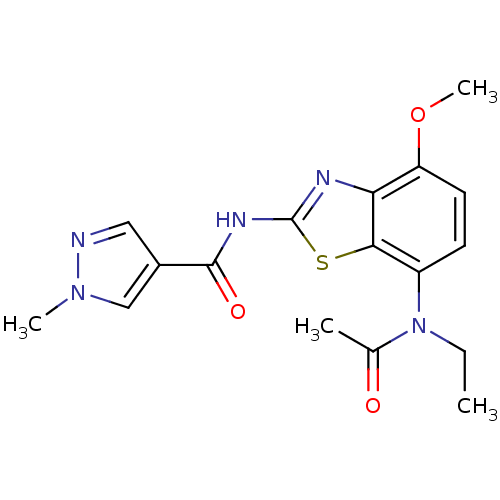
BDBM50321521 CHEMBL1171163::N-(7-(N-ethylacetamido)-4-methoxybenzo[d]thiazol-2-yl)-1-methyl-1H-pyrazole-4-carboxamide
SMILES CCN(C(C)=O)c1ccc(OC)c2nc(NC(=O)c3cnn(C)c3)sc12
InChI Key InChIKey=FMPFRHFXEJRKAA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50321521
Found 3 hits for monomerid = 50321521
Affinity DataKi: 19nMAssay Description:Displacement of [3H]ZM241385 from human adenosine A2B receptor expressed in CHO cells after 1 hr by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in CHO-K1 cells after 1 hr by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Antagonist activity at human adenosine A2B receptor expressed in CHO cells assessed as inhibition of adenosine-induced cAMP productionMore data for this Ligand-Target Pair