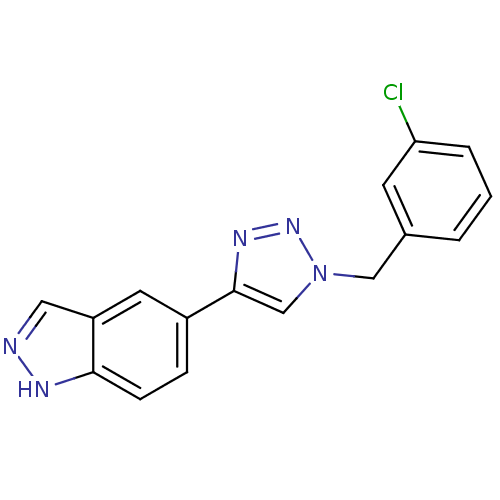
BDBM50337317 5-(1-(3-chlorobenzyl)-1H-1,2,3-triazol-4-yl)-1H-indazole::CHEMBL1682348
SMILES Clc1cccc(Cn2cc(nn2)-c2ccc3[nH]ncc3c2)c1
InChI Key InChIKey=WVZWFOGYDXWKPT-UHFFFAOYSA-N
Data 5 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50337317
Found 5 hits for monomerid = 50337317
Affinity DataKi: 23nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Inhibition of Aurora kinase 2 after 1 hr using biotinylated kemptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1.45E+3nMAssay Description:Inhibition of JAK2 after 1 hr using biotinylated PDKtide as substrateMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 1.31E+4nMAssay Description:Inhibition of KDR after 1 hrMore data for this Ligand-Target Pair