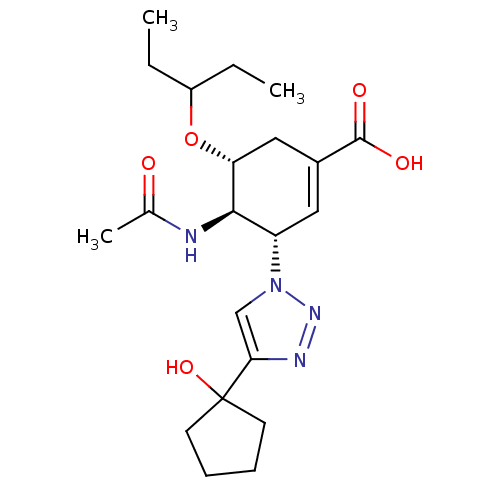
BDBM50343688 (3S,4R,5R)-4-Acetamido-5-(1-ethylpropoxy)-3-[4-(1-hydroxycyclopentyl)[1,2,3]triazol-1-yl]cyclohex-1-ene-1-carboxylic Acid::(3S,4R,5R)-4-acetamido-3-(4-(1-hydroxycyclopentyl)-1H-1,2,3-triazol-1-yl)-5-(pentan-3-yloxy)cyclohex-1-enecarboxylic acid::CHEMBL1270329
SMILES CCC(CC)O[C@@H]1CC(=C[C@@H]([C@H]1NC(C)=O)n1cc(nn1)C1(O)CCCC1)C(O)=O
InChI Key InChIKey=VTDCKCKCZWKRBP-YQVWRLOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50343688
Found 3 hits for monomerid = 50343688
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of human NEU3 using 4MU-NA as substrate after 1 hr by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of influenza A nuraminidase N1More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of human NEU4 using 4MU-NA as substrate after 1 hr by fluorescence assayMore data for this Ligand-Target Pair