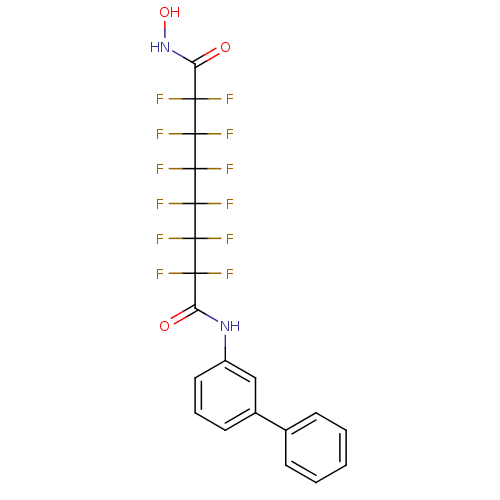
BDBM50361262 CHEMBL1934904
SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1cccc(c1)-c1ccccc1
InChI Key InChIKey=RFDGYNNXFQVRFM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50361262
Found 7 hits for monomerid = 50361262
TargetHistone deacetylase 4(Homo sapiens (Human))
University Of Applied Sciences Darmstadt
Curated by ChEMBL
University Of Applied Sciences Darmstadt
Curated by ChEMBL
Affinity DataKi: >5.00E+4nMAssay Description:Inhibition of human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m...More data for this Ligand-Target Pair
TargetHistone deacetylase 5(Homo sapiens (Human))
University Of Applied Sciences Darmstadt
Curated by ChEMBL
University Of Applied Sciences Darmstadt
Curated by ChEMBL
Affinity DataKi: >5.00E+4nMAssay Description:Inhibition of human HDAC5 using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 m...More data for this Ligand-Target Pair
Affinity DataKd: 1.50E+4nMAssay Description:Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assayMore data for this Ligand-Target Pair
Affinity DataKd: 6.00E+4nMAssay Description:Inhibition of recombinant human HDAC8 using Boc-L-Lys(trifluoroacetyl)-MCA as substrate by fluorogenic enzymatic assayMore data for this Ligand-Target Pair
Affinity DataKd: 3.00E+4nMAssay Description:Inhibition of recombinant human HDAC1 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assayMore data for this Ligand-Target Pair
TargetHistone deacetylase-like amidohydrolase(Alcaligenes sp. (strain DSM 11172) (Bordetella sp....)
University Of Applied Sciences
Curated by ChEMBL
University Of Applied Sciences
Curated by ChEMBL
Affinity DataKd: 450nMAssay Description:Inhibition of Bordetella FB188 HDAH using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assayMore data for this Ligand-Target Pair
Affinity DataKd: 1.40E+4nMAssay Description:Inhibition of recombinant human HDAC7 using Boc-L-Lys(trifluoroacetyl)-MCA as substrate by fluorogenic enzymatic assayMore data for this Ligand-Target Pair